Rifabutin liposomes: a novel nanotechnological strategy for effective treatment of systemic methicillin-resistant staphylococcus aureus infections
Dr. M. Manuela Gaspar1) – University of Lisbon, Portugal
People involved
Jacinta Oliveira Pinho (Post-doc fellow sponsored by the PRC) - Research Institute for Medicines (iMed.ULisboa) Faculty of Pharmacy, University of Lisbon, Lisboa, Portugal
Lurdes Gano PhD and Célia Fernandes PhD, Researchers at Campus Technological Nuclear, Instituto Superior Técnico, University of Lisbon, Lisboa Portugal
Sandra Aguiar PhD, Researcher at Faculdade de Medicina Veterinária, University of Lisbon, Lisboa, Portugal
Frederico Aires da Silva PhD, Assistant Researcher at Faculdade de Medicina Veterinária, University of Lisbon, Lisboa, Portugal
Sandra Pinto PhD, Researcher, Instituto Superior Técnico, University of Lisbon, Lisboa, Portugal
Magda Ferreira, PhD Student at Research Institute for Medicines (iMed.ULisboa) Faculty of Pharmacy, University of Lisbon, Lisboa, Portugal
Abstract
Staphylococcus aureus, and in particular the methicillin-resistant (MRSA) strains are one of the most infectious bacterial pathogens worldwide, renowned for their ability to evade the immune system causing systemic infections. After entering the bloodstream, MRSA can cause metastatic or complicated infections such as infective endocarditis or sepsis, being associated with poor clinical outcomes and exhibiting high rates of morbidity and mortality. The incidence of systemic MRSA worldwide ranges from 20 to 50 per 100,000 people for countries with low and high incidence rates, respectively with a mortality associated of 20% within 30 days.1) Despite novel anti-MRSA drugs, sub-therapeutic levels of the antibiotics at intracellular infection sites due to unfavorable pharmacokinetics are in the origin of the emergence of drug resistance and consequently of antibacterial failure in the clinic thus representing a major reason of concern.
The present project aims to validate in preclinical studies an effective lipid-based nano-formulation against systemic infections caused by MRSA. Rifabutin (RFB) was the chosen antibiotic and nanoliposomes the lipid-based system. The rational for selecting RFB is based on its antibacterial potential against MRSA and their incorporation in nanoliposomes is due to the well-known ability of liposomal formulations, depending on their lipid compositions, to enhance the mean residence time in the bloodstream of the loaded compound, leading to enhanced accumulation in infected areas.2) In addition, liposomes can improve the interaction with bacterial membranes thus enabling the achievement RFB concentrations intracellularly at infected sites at much higher concentrations in comparison to the use of antibiotic in the free form.
Preliminary in vitro studies have already demonstrated the potential of RFB over other antibiotics such as vancomycin or levofloxacin. RFB was successful and efficiently encapsulated in pre-formed unloaded liposomes with excellent stability characteristics, using an active loading method. In addition, RFB incorporated in liposomes displayed even a more pronounced antibacterial effect, particularly for a negatively charged lipid-based nano-formulation.3)
We propose to establish a systemic MRSA murine model of infection and evaluate the in vivo fate of the developed RFB liposomal formulations after i.v. administration. For that, dual labelling of RFB and liposomes will be accomplished with 99mTc (or 125I) and 111In, respectively.4)5) This procedure will enable to follow, simultaneously, the in vivo fate of both RFB and liposome, and consequently, elucidate the stability of the RFB liposomal formulations and accumulation at different organs over time as well as in the blood. These studies will be crucial for selecting the best treatment schedules to be performed in the systemic murine MRSA model of infection. Vancomycin administered in the free form will be used as positive control.
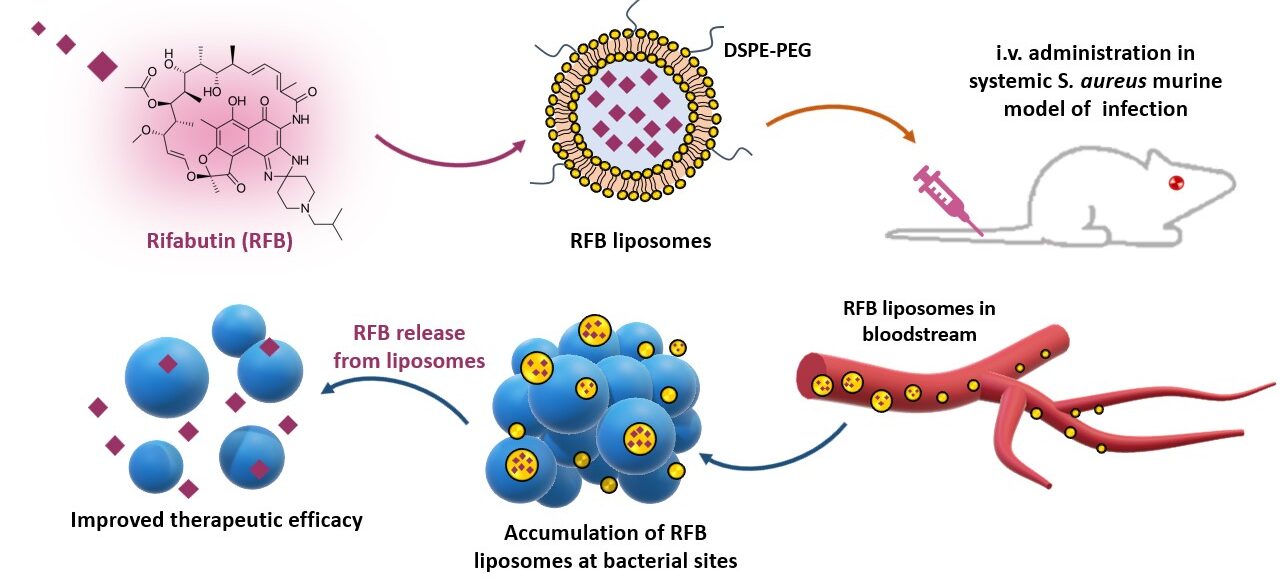
Figure 1. Schematic representation of the workflow of this project.
Benefit for the community
The obtained results will contribute to improve the therapies against one of the most problematic bacterial infections threatening human health – the systemic MRSA infections.
Visit the supervisors lab
Risk factors of treatment failure and 30-day mortality in patients with bacteremia due to MRSA with reduced vancomycin susceptibility
Sci. Rep. 8, 7868
| PubMed |
Antibiotic-nanomedicines: facing the challenge of effective treatment of antibiotic-resistant respiratory tract infections
Future Microbiol. 13, 1683-1692
| PubMed |
Liposomes as a Nanoplatform to Improve the Delivery of Antibiotics into Staphylococcus aureus Biofilms
Pharmaceutics 13, 321
| MDPI |
Enzymosomes with surface-exposed superoxide dismutase: in vivo behaviour and therapeutic activity in a model of adjuvant arthritis
J. Controlled Release 117, 186-195
| PubMed |
Radiolabeled block copolymer micelles for image-guided drug delivery
Int. J. Pharm. 515, 692-701
| PubMed |
Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance
Molecules 26, 2047
| PubMed |
Preclinical validation of a new hybrid molecule loaded in liposomes for melanoma management
Biomed. Pharmacother. 157, 114021
| PubMed |
A step forward on the in vitro and in vivo assessment of a novel nanomedicine against melanoma
Int. J. Pharm. 640, 123011
| PubMed |
Liposomal Rifabutin—A Promising Antibiotic Repurposing Strategy against Methicillin-Resistant Staphylococcus aureus Infections
Pharmaceuticals 17, 470
| MDPI |


