The unique and different types of phospholipids
There are different types of phospholipids. In this article, we give an answer to the question "What are phospholipids?" and explain the various phospholipid types.
What are phospholipids?
Phospholipids are unique and versatile molecules. They are of natural occurrence and the main components in cellular membranes. Arranged as a lipid bilayer, phospholipids play a significant role in the structure and functionality of biological membranes. In addition, they are involved in several cellular processes and used as excipient in various pharmaceutical formulations. Phospholipids are amphiphilic, which means they "love" both water and fat, and consist of a hydrophilic as well as a lipophilic moiety.
Phospholipids are esters of glycerol. The sn-1 and sn-2 positions of the glycerol backbone are esterified with fatty acids of varying length and degree of saturation (illustrated in the symbolic presentations of phospholipids by yellow sticks). The remaining sn-3 position is esterified with phosphoric acid which, in turn, is esterified with a short-chain alcohol of varying chemical structure forming the hydrophilic headgroup of the phospholipid (illustrated by a blue ball, see Figure 1).1) The systematic designation of, for example, phosphatidic acid (PA) is 1,2-diacyl-sn-glycero-3-phosphate, where “sn” means stereospecific numbering or stereo-specifically numbered, i.e., the carbon atom that appears on top in the Fischer projection showing a vertical carbon chain with the hydroxyl group at carbon-2 to the left is designated as C-1.2)
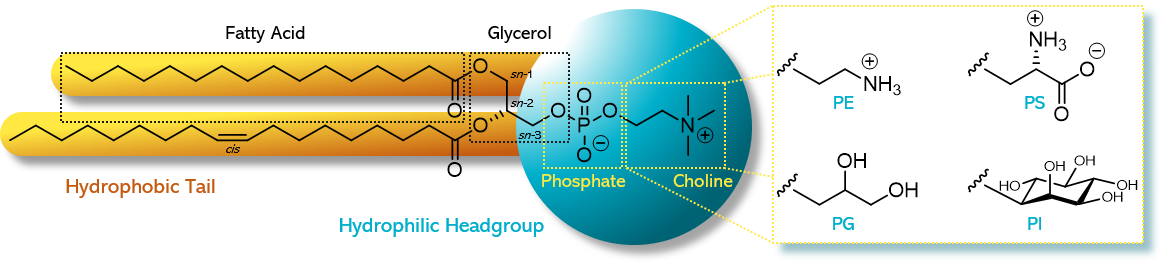
Figure 1. Chemical structure of a phospholipid as exampled by 1-palmitoyl-2-oleoyl-sn-glycero-3phosphocholine (POPC) and the different alternative headgroups varying in the type of alcohol: phosphatidylethanolamine (PE) with ethanolamine, phosphatidylglycerol (PG) with glycerol, phosphatidylserine (PS) with serine, and phosphatidylinositol (PI) with inositol. Figure adapted from Drescher S, van Hoogevest P, 2020. The Phospholipid Research Center: Current Research in Phospholipids and their Use in Drug Delivery, Pharmaceutics 12, 1235.1)
Classification of phospholipids
Depending on the structure of this alcohol, different types of phospholipids comprise, for example, phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), or phosphatidylserine (PS).3) The four major phospholipids predominant in the plasma membrane of many mammalian cells are PC, PE, PS, and sphingomyelin. The specific and non-random distribution of substituents over the positions sn-1, sn-2, and sn-3 of the glycerol introduces chirality. Depending on the structure of the polar headgroup and pH of the surrounding medium, PE and PC are zwitterionic and have a neutral charge at pH 7, whereas PG, PI, and PS are negatively charged at this pH value (see Figure 2).
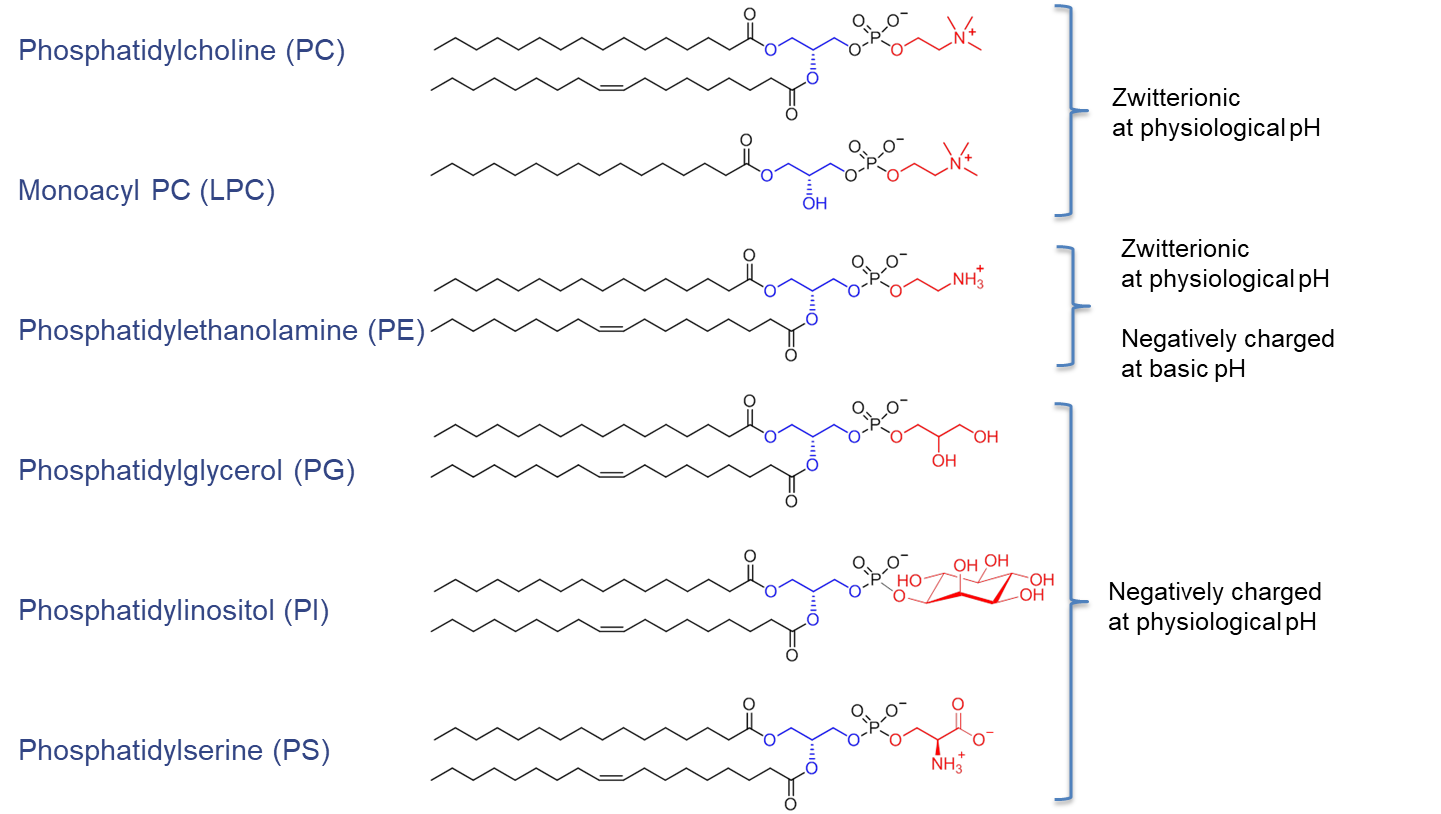
Figure 2. Chemical structure of phospholipids (blue, glycerol; red, alcohol) and their net charge at physiological pH value. Figure adapted from van Hoogevest P, Tiemessen H, Metselaar JM, Drescher S, Fahr A, 2021. The Use of Phospholipids to make Pharmaceutical Form Line Extensions, Eur. J. Lipid Sci. Technol., 2000297.4)
Nomenclature of phospholipids
The phospholipid may have two esterified fatty acids and are called diacyl-phospholipids, whereas phospholipids with one fatty acid are called monoacylphospholipids or lyso-phospholipids.
In scientific literature, synthetic phospholipids are named in an abbreviated way according to “number of the position of fatty acids—type of fatty acid—phosphatidyl—alcohol”. The number of fatty acids could be 'mono' or 'di', the fatty acids are described as, e.g., oleoyl or palmitoyl (coming from oleic acid and palmitic acid, respectively), 'phosphatidyl' describes the backbone of the phospholipid molecule which encompasses glycerol, further esterified with one or two fatty acid and a phosphate group, and finally the alcohol is mentioned as described above. The International Union of Pure and Applied Chemistry (IUPAC) instead uses “glycero—phospho—alcohol”. Examples are POPC, 1-palmitoyl-2-oleoylphosphatidylcholine (IUPAC: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) or DOPE, 1,2-dioleoylphosphatidylethanolamine (IUPAC: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine).
Since the number, type and position of the fatty acid as well as the type of headgroup can be varied modularly, there is a very large number of different phospholipids in nature. Depending on the origin of the natural phospholipids (plant or animal sources), the composition of fatty acids differs.
For more information check out our phospholipid publications.
Phospholipid, Lecithin, Phosphatidylcholine, what is what?
However, one must be aware of the different meanings and uses of the term “phosphatidylcholine” and “lecithin”. According to the United States Pharmacopeia (USP), lecithin is described as “a complex mixture of acetone-insoluble phosphatides (i.e., phospholipids), which consist chiefly of PC, PE, PI, and PA, present in conjunction with various amounts of other substances such as triglycerides, fatty acids, and carbohydrates, as separated from the crude vegetable oil source. It contains not less than 50% of acetone-insoluble matter.” Unfortunately, especially in the American literature, lecithin is also used as a synonym of phosphatidylcholine, which is the pure compound. Descriptions of the phospholipid component in products as “lecithin” leaves open which lipid is used. It is therefore recommend using for natural phospholipids the term “lecithin” only when the product contains less than 80% by weight total phospholipids and the term “phospholipid” when the product contains 80–100% by weight phospholipids (e.g., PC, PE); the specific phospholipid should be mentioned when the product contains more than 90% by weight of that specific phospholipid.1)
You want to know more?
Please use the following links:
What is the difference between natural and synthetic phospholipids?
What are the phospholipids benefits?
What phospholipid aggregates are formed?
Which pharmaceutical formulations can be made?
The Phospholipid Research Center: Current Research in Phospholipids and their Use in Drug Delivery
Pharmaceutics 12, 1235
| PubMed |
Nomenclature of phosphorus-containing compounds of biochemical importance (Recommendations1976)
Proc. Natl. Acad. Sci. USA 74, 2222
| Pubmed |
The Use of Phospholipids to make Pharmaceutical Form Line Extensions
Eur. J. Lipid Sci. Technol., 2000297
| Wiley |


