Natural versus synthetic phospholipids
Phospholipids are ubiquitous molecules that are an essential component of biological membranes and participate in countless cellular processes. Besides that, they play a prominent role in the pharmaceutical industry as phospholipids can be technologically used as, for example, an emulsifier, wetting agent, solubilizer, and agent in the formation of liposomes and mixed micelles, and many more pharmaceutical formulations. In principal, one can distinguish between natural and synthetic phospholipids. Here you will find out more about the similarities and differences between both phospholipid types.
Natural Phospholipids
Natural phospholipids can be obtained from vegetable sources such as soybeans, sunflower, rape (canola) seed, wheat germ, and flax seed, and animal material such as hen egg yolk, milk, or krill. The phospholipid and fatty acid profiles of the various lecithins, that means, which fatty acids are connected to the glycerol and which headgroup is attached, depend on the raw material sources. The composition of de-oiled vegetable lecithin from variable sources and egg lecithin are provided as example in Table 1.1)2)
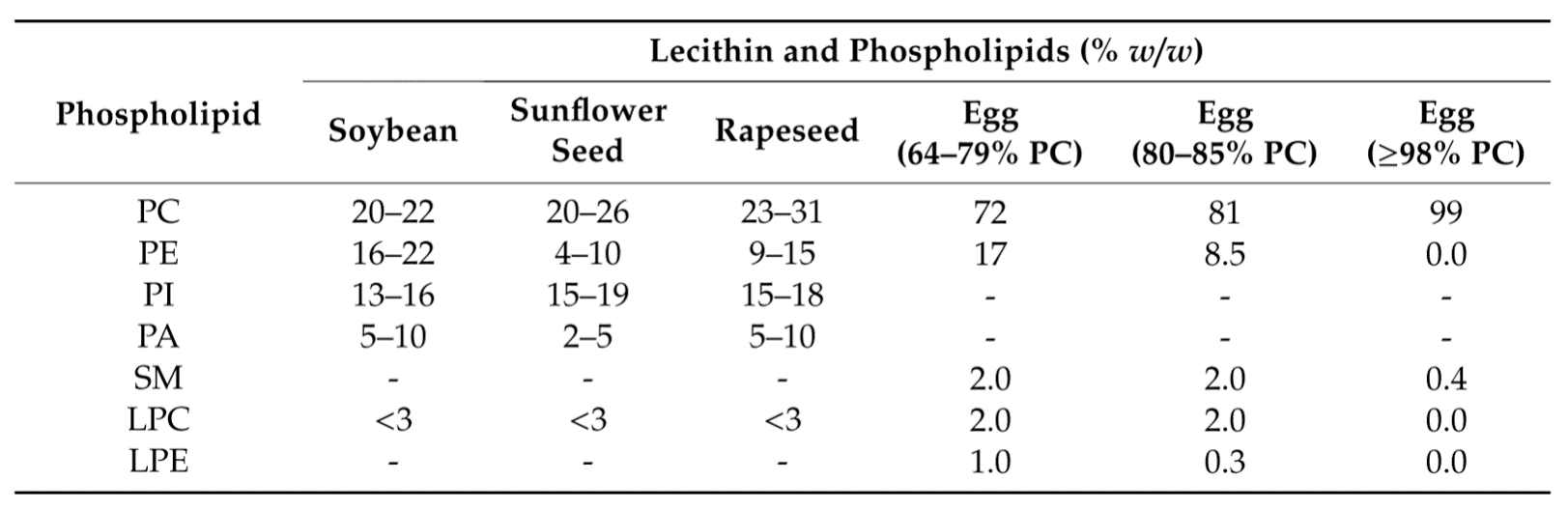
Table 1. Phospholipid composition of vegetable de-oiled lecithins and egg phospholipids of different PC contents, respectively. Data taken from van Hoogevest P, Wendel A, 2014. The use of natural and synthetic phospholipids as pharmaceutical excipients, Eur. J. Lipid Sci. Technol. 116, 1088.2)
Besides phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylinositol (PI), other components can also occur in natural lecithins. These are namely sphingomyelin (SM), phosphatic acid (PA), and minor amounts of phospholipids containing only one acyl chain in the sn-1 position, namely lyso-phosphatidylcholine (LPC) and lyso-phosphatidylethanolamine (LPE). In all cases, PC is the main phospholipid component. To convert these materials into high-quality excipients, meeting pharmacopeial and regulatory requirements for parenteral and other specific formulations, extraction and chromatography procedures are applied.
The origin of phospholipids defines the fatty acid composition
The fatty acid composition of typical batches of vegetable de-oiled lecithins and egg phospholipid of different PC content, respectively, is given in Table 2.2) The corresponding fatty acids are listed in their short notation, with the first digit indicating the number of carbon atoms and the second digit the numbers of cis-double bonds:
- C14:0, myristic acid (tetradecanoic acid)
- C16:0, palmitic acid (hexadecenoic acid)
- C18:0, stearic acid (octadecanoic acid)
- C18:1, oleic acid (octadecenoic acid)
- C18:2, linoleic acid (octadecadienoic acid)
- C18:3, α-linolenic acid (ALA, octadecatrienoic acid)
- C20:0, arachidic acid (eicosanoic acid)
- C20:4, arachidonic acid (ARA, eicosatetraenoic acid)
- C22:0, behenic acid (docosanoic acid)
- C22:4, docosatetraenoic acid
- C22:5, docosapentaenoic acid (DPA)
- C22:6, cervonic acid (docosahexaenoic acid, DHA)
The presence of the polyunsaturated fatty acids (PUFAs) C20:4 and C22:6 is typical for hen egg yolk phospholipids.
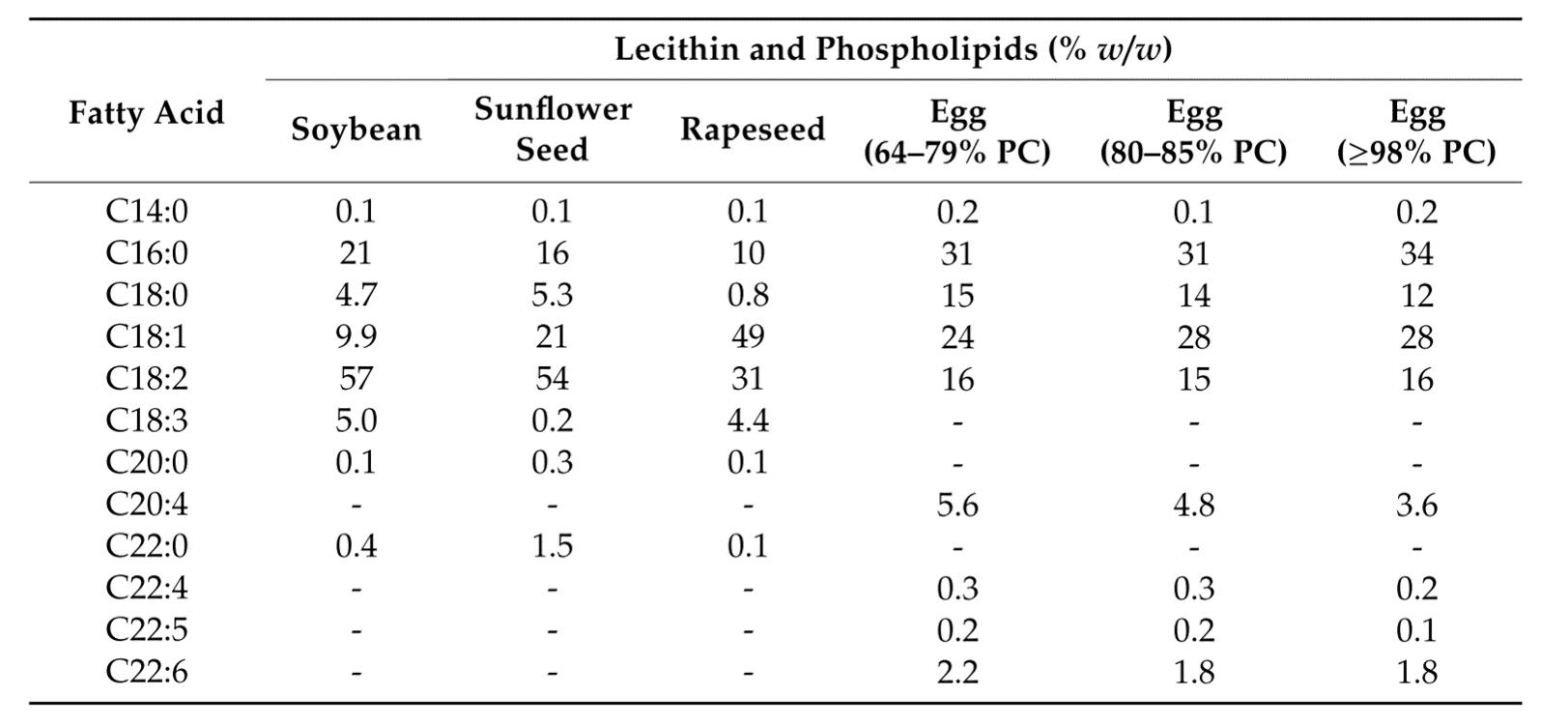
Table 2. Fatty acid composition of vegetable de-oiled lecithins and egg phospholipids of different PC contents, respectively. Data taken from van Hoogevest P, Wendel A, 2014. The use of natural and synthetic phospholipids as pharmaceutical excipients, Eur. J. Lipid Sci. Technol. 116, 1088.2)
Synthetic phospholipids
Due to the presence of unsaturated fatty acids in natural phospholipids, the liquid crystalline to gel phase transition temperature (Tm) is below 0 ◦C. These phospholipids are at ambient temperature in the liquid crystalline state (Lα phase). They form upon hydration flexible structures/mesophases,3) suitable for specific pharmaceutical technological applications. In some formulations, however, when more physically stable liposomes with increased stability in blood plasma or phospholipids with more powder-like properties are required, phospholipids with higher Tm are preferred.4) Phospholipids with saturated fatty acids possess these properties. They can be obtained by hydrogenation of natural phospholipids with unsaturated fatty acids,5) resulting in, for example, HSPC (hydrogenated soybean PC). The progress of the hydrogenation can be followed by monitoring the iodine value, which is a measure of the degree of unsaturation of fatty substances.
Semi-synthetic phospholipids
Nowadays, alternative biochemical synthesis routes via enzyme-catalyzed reactions serve as a viable alternative to organic-chemical synthesis steps. In that respect, the use of enzymes for phospholipid modification has moved quickly in recent years, not only in academic research but also in industry.6) The fast-growing use of enzymes for polar lipid modification arises from factors such as milder reaction conditions, less environmental pollution, better specificity for improved quality, and higher efficiency of reactions. Specific enzymes are suitable for different modification purposes to modify/synthesize phospholipids.
For acyl modifications, natural enzymes such as phospholipase A1 and A2 (PLA1 and PLA2), which selectively cleave the fatty acid in sn-1 and sn-2 leading to lyso-phospholipids, can be used. Phospholipase B (PLB) is an enzyme with a combination of both PLA1 and PLA2 activities: it can cleave acyl chains from both the sn-1 and sn-2 positions. Phospholipase C (PLC) can hydrolyze the bond between the glycerol oxygen and the phosphate group, leading to the formation of diacylglycerol and phosphocholine. Unfortunately, this enzyme family is only active for the hydrolysis reaction and not for the reformation process. Therefore, PLC and PLB are not used industrially. Finally, phospholipase D (PLD) is the only potential enzyme for polar group modification (see Figure 1). This enzyme cleaves the bond between the phosphate and the choline. Examples of enzyme-modified (“semi-synthetic”) phospholipids prepared from natural PC are lyso-PC, soybean PE, soybean PG, egg PG, and their saturated analogs.
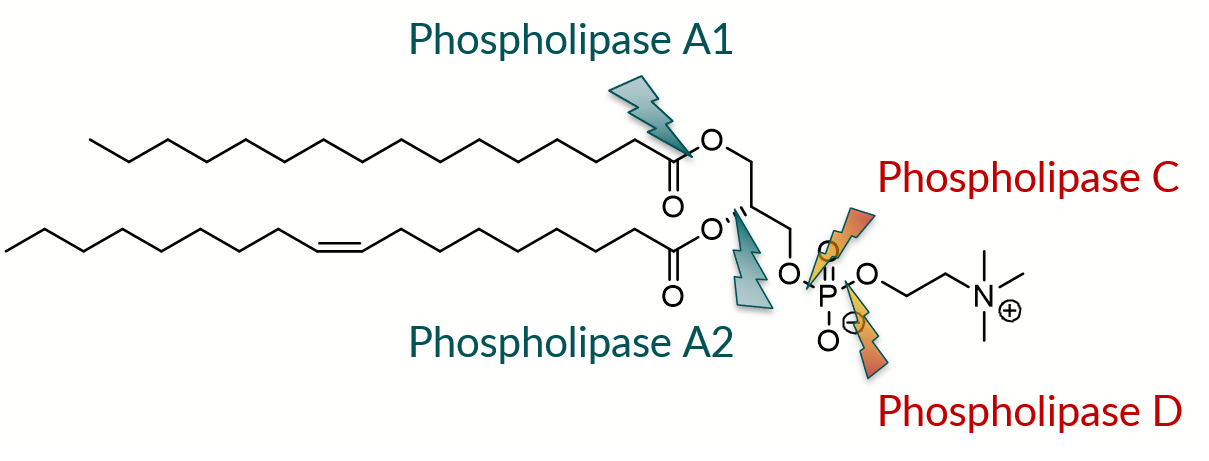
Figure 1. The use of phospholipases to produce semi-synthetic phospholipids.
Full-synthetic phospholipids
To study more mechanistic biochemical or biophysical aspects of phospholipids at the molecular level in natural environments or in model membranes, various synthetic approaches to chemically well-defined phospholipids have been developed. These “full-synthetic” phospholipids are then homogeneous with respect to the polar headgroup and fatty acid composition.2)3)6)7)
For the preparation of “full-synthetic” phospholipids, several starting compounds are conceivable. The classical approach to synthesize diacyl-PCs is to start from D-mannitol. However, this synthetic method is lengthy, requires toxic chemicals and solvents in large excess, and reaction intermediates may be unstable and subject to partial racemization.8)9) A simpler method to synthesize symmetrical glycerophospholipids, which are phospholipids bearing two identical fatty acids, is a method starting from (R) or (S) glycidyl tosylates.10) The enantiomerically pure glycidyl derivative is, however, expensive, making large scale syntheses of glycerophospholipids impractical.3)
Another route to produce phospholipids with defined fatty acids is to start from glycerophosphocholine (GPC), which is also termed sn-glycero-3-phosphocholine, l-α-glyceryl-phosphorylcholine, α-GPC, or choline alfoscerate. GPC can be synthesized in an enantioselective manner using a biotransformation procedure based on the phosphorylation of glycerol by adenosine triphosphate (ATP) catalyzed glycerol kinase11) or by means of alkaline hydrolysis from natural PC maintaining the natural stereoisomeric structure. Starting from the latter, symmetrical phospholipids can be synthesized in a one step process using activated acyl derivatives, such as acylimidazolides or anhydrides,7)12) and various coupling reagents such as dicyclohexylcarbodiimide (DCC) in combination with 4-(dimethylamino)pyridine (DMAP)13) or 2-methyl-6-nitrobenzoic anhydride (MNBA).14) For the further synthesis from GPC to asymmetrical, i.e., mixed fatty acid chain phospholipids, organic chemical methods as well as enzymatic procedures can be applied.
Industrial production of phospholipids
To exemplify the production process of natural phospholipid excipients starting from plant oil, the production process of soybean lecithin is shown. First, crude soybean lecithin is isolated by degumming of the crude soybean oil as obtained by extraction from soybean (see Figure 2).15) The crude soybean lecithin obtained serves as starting material for production at a large scale of soybean lecithin fractions with higher PC content. These fractions are obtained in high yields by extraction methods using non-toxic solvents followed by chromatographic purification procedures and appropriate solvent removal methods. By selecting appropriate sequential extraction and chromatography methods, several lecithin fractions differing in PC content from 20 to 80% up to pure PC (≥98%) can be reproducibly achieved. This procedure applies to soybean oil and all vegetable oils used to produce lecithin, and high-purity phospholipids/PC. Furthermore, egg phospholipids are isolated from hen egg yolk with similar extraction and chromatography methods as for soybean lecithin.
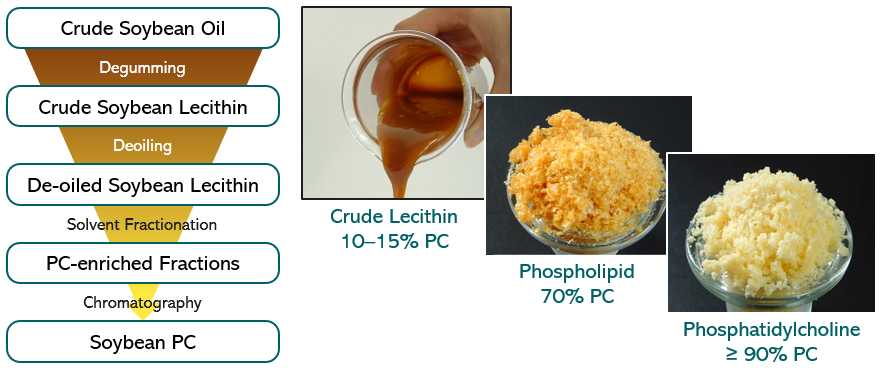
Figure 2. (Left) Flow chart of isolation process steps of soybean phosphatidylcholine derived from crude soybean oil. (Right) Visual appearance of soybean lecithin/PC fractions with variable PC content. Figure adapted from Drescher S, van Hoogevest P, 2020 The Phospholipid Research Center: Current Research in Phospholipids and their Use in Drug Delivery, Pharmaceutics 12, 1235.1)
You want to know more?
Please use the following links:
What types of phospholipids are there?
What is the occurrence and benefit of phospholipids?
What phospholipid aggregates are formed?
Which pharmaceutical formulations can be made?
The Phospholipid Research Center: Current Research in Phospholipids and their Use in Drug Delivery
Pharmaceutics 12, 1235
| PubMed |
The use of natural and synthetic phospholipids as pharmaceutical excipients
Eur. J. Lipid Sci. Technol. 116, 1088
| PubMed |
Stability of small unilamellar liposomes in serum and clearance from the circulation: the effect of the phospholipid and cholesterol components
Life Sci. 30, 2123
| PubMed |
Chemical Reactions of Phosphatides
in: Lecithins (Eds: Szuhaj, BF, List, GR), American Oil Chemists’ Society: Champaign, IL, USA, Volume 1, pp. 213–246
| Google Scholar |
Chapter 3 - Enzymatic modification of phospholipids and related polar lipids
in: Phospholipid Technology and Applications (Ed: Gunstone, FD), Woodhead Publishing, Bridgewater, pp. 41-82
| Google Scholar |
An enantioselective synthesis of platelet-activating factors, their enantiomers, and their analogues from D- and L-tartaric acids
Chem. Pharm. Bull. 33, 572
| PubMed |
Synthesis of monoacid 2,3-diacyl-sn-glycerols via 1,6-ditrityl-d-mannitol
Chem. Phys. Lipids 27, 185
| ScienceDirect |
Glycerol Kinase: Synthesis of Dihydroxyacetone Phosphate, sn-Glycerol-3-Phosphate, and Chiral Analogues
J. Am. Chem. Soc. 107, 7019
| ACS |
An improved method for the preparation of unsaturated phosphatidylcholines: acylation of sn-glycero-3-phosphorylcholine in the presence of sodium methylsulfinylmethide
J. Lipid Res. 18, 548
| PubMed |
Detailed Comparison of Deuterium Quadrupole Profiles between Sphingomyelin and Phosphatidylcholine Bilayers
Biophys. J. 106, 631
| PubMed |
in: Kirk-Othmer Encyclopedia of chemical Technology (Eds: R. E. Kirk, D. F. Othmer)
John Wiley & Sons, New York, Chichester, Weinheim, Brisbane, Singapur, Toronto, pp. 191-210
| Google Scholar |


