The different pharmaceutical formulations with phospholipids excipients
There are many pharmaceutical formulations that contain phospholipids as excipients. You can learn more about these formulations: liposomes, emulsions or the well-known lipid nanoparticles in the following article.
Which formulations can be made with phospholipids?
Phospholipids, natural as well as synthetic, are broadly used excipients in pharmaceutical technology as wetting agents, emulsifiers, and builders of different lipid mesophases such as closed lipid vesicles (liposomes), micelles, inverted micelles, etc.1) These functional properties are applied in many types of pharmaceutical formulations such as suspensions, different types of emulsions, solid dispersions, lipid nanoparticles (LNPs), drug/phospholipid complexes, etc.2)3) Such formulation types can be used in any dosage forms, such as tablets, capsules, injections, creams, etc.
With respect to their physiological role, phospholipids possess a very low toxicity profile and are suitable for any route of administration, namely parenteral, oral, and topical (dermal) and inhalation. Regarding the versatility, phospholipids are superior excipients compared to synthetic non-biodegradable polymers, which are not appropriate to be used for every administration route and which are, by definition, non-physiological.
Which phospholipid for which application?
In case natural phospholipids derived from hen egg yolk or soybean are selected, attention should be paid to the minimal quality in phospholipid content, which depends on the administration route. For oral and dermal administration, natural phospholipids with at least 45% phosphatidylcholine (PC) can be used, whereas for parenteral use, phospholipid fractions with at least 70% PC are typical. For specific injectable, high-tech parenteral products, more expensive, synthetic, and chemically well-defined phospholipids of high purity may be the best choice, whereas for topical and oral administration (and of course other parental applications), cost effective natural phospholipids are preferable, and approved in marketed drug products.
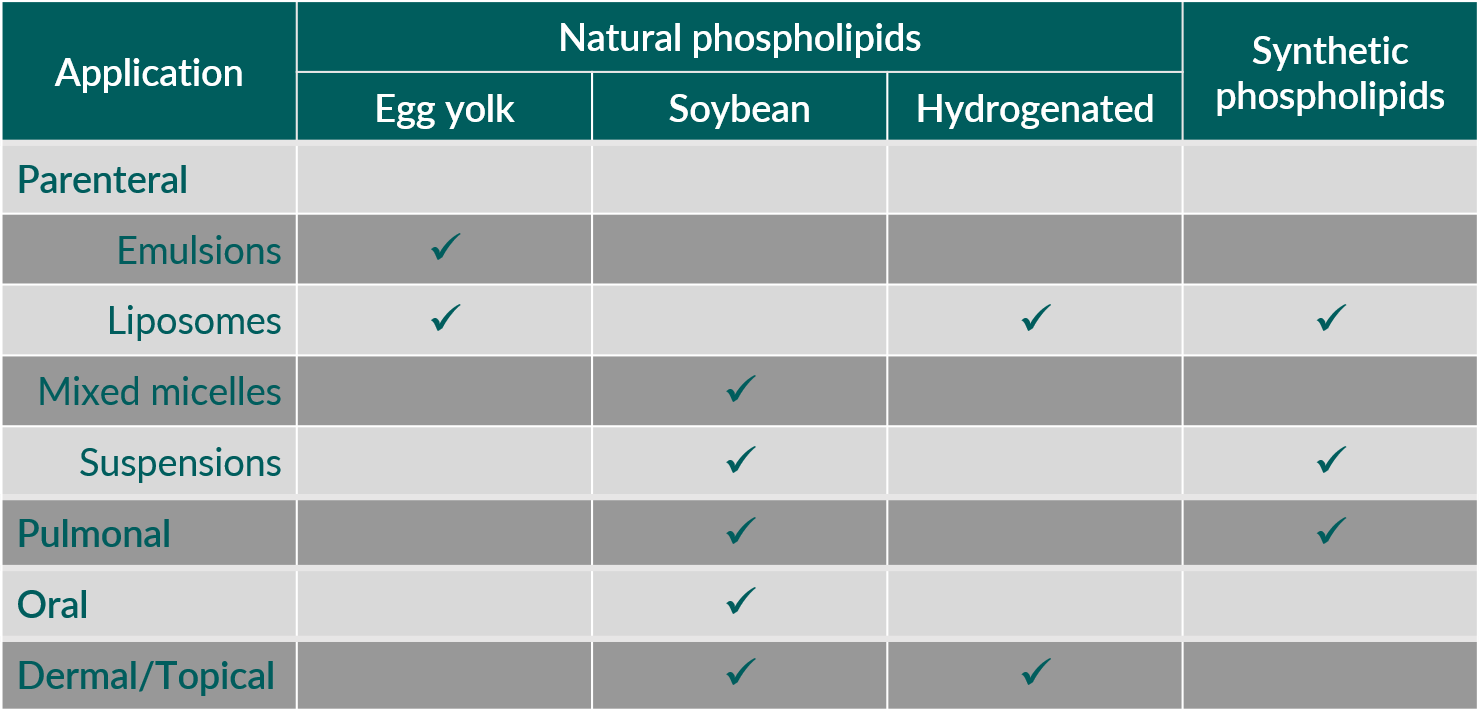
Table 1. Phospholipids in pharmaceutical applications: Which phospholipid is used?
The categories of pharmaceutical formulations, which are of interest to study the prevalence of natural and synthetic phospholipids, respectively, are products used for parenteral administration.1) These are mainly liposomes, oil-in-water (o/w) emulsions, mixed micelles for intravenous (i.v.) use and slow release, vaccine vehicles, and drug suspensions for intramuscular (i.m.) and subcutaneous (s.c.) administration.
Liposomal products for i.v. administration are formulated with synthetic as well as natural phospholipids. Liposomes can also be used as vehicles for slow release after local parenteral administration, for example at the surgical site, epidural or intrathecal. In this case, mainly synthetic phospholipids are applied. Egg phospholipids are present in o/w emulsions for parenteral nutrition and as carriers for oil-soluble drug substances.4)
In mixed micellar formulations, including phospholipids and different cholate salts, exclusively soybean phospholipids are used. Here, the phospholipids act as solubilizer for poorly water-soluble substances or as active principles (for example soybean PC), through the presence of polyunsaturated fatty acids (PUFAs) for treatment of liver disorders.5)
Considering products comprising phospholipids for pulmonary administration, natural as well as synthetic phospholipids are applied. Most of these products are administered in the treatment of respiratory distress syndrome in infants6) or bacterial lung infections.
Regulatory and safety aspects
Natural phospholipids are generally well-known to regulatory authorities. Their track record as excipients with very high tolerability and biocompatibility is outstanding. The World Health Organization (WHO) places no limit on the oral intake of lecithin. Furthermore, no limit for the value of acceptable daily intake (ADI) for lecithin as a food additive is given. The pediatric oral use of phospholipids (soybean) is in general allowed, of course with precautions for soybean allergy. Alternatively, sunflower phospholipids, with no allergy warnings, can be used. After parenteral administration, egg and soybean phospholipids—unsaturated and saturated variations—are very well tolerated.
The European Commission declares that lecithin is a food additive (E322) “generally permitted for use in foodstuffs”. Furthermore, no ADI value has been fixed for lecithin in Europe; the material may be used in food quantum satis. The US Food and Drug Administration (FDA) assigned the "generally recognized as safe" (GRAS) affirmation for lecithin and enzyme-modified lecithin.
Synthetic phospholipids are not mentioned in pharmacopoeias yet. Just like hydrogenated natural phospholipids they can be found in the Inactive Ingredient Guide by the FDA Center for Drug Evaluation and in the relevant reference documents of FDA and EMA mentioned above. The quality and safety have to be addressed in the application documentation of the drug product.1)
Liposomes
Focusing on parenteral applications, liposomes and liposomal formulations of active pharmaceutical ingredients (API) play an outstanding role, which is due to but not restricted to the introduction of Doxil®. Doxil (doxorubicin HCl for injection) was the first nano-drug approved by the U.S. Food and Drug Administration (FDA) in 1995.7)
Per definition, liposomes are aggregates of phospholipids in water, in which the phospholipids are arranged in a bilayer membrane surrounding one or more aqueous compartments.
Examples of parenteral products with liposomes are provided in the following Table 2.
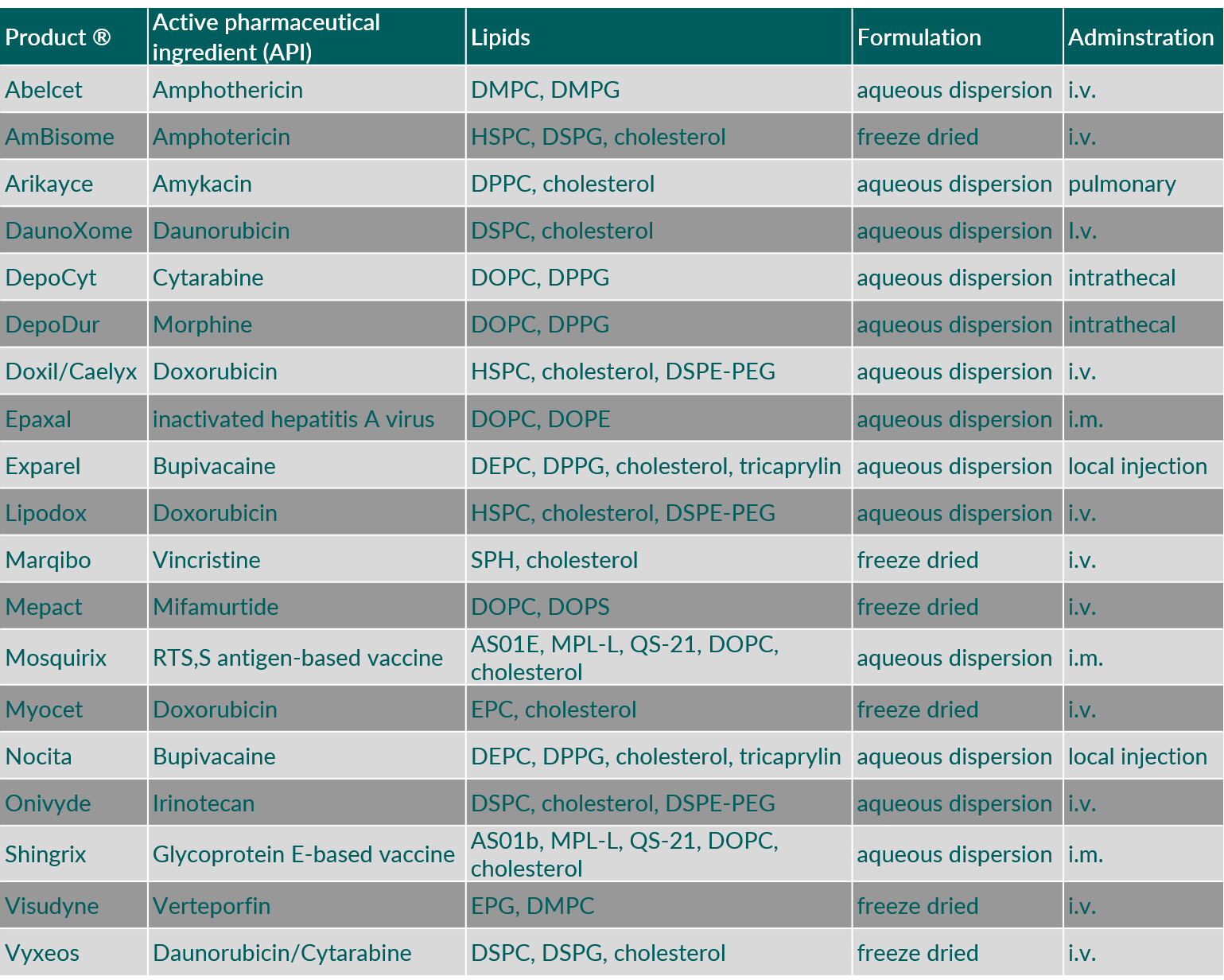
Table 2. Liposomal formulations approved in EU and US. (Abbreviations: DEPC, 1,2-dierucoyl-sn-glycero-3-phosphocholine; DMPC, 1,2-dimyristoyl-sn-glycero-3-phosphocholine; DMPG, 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOPS, 1,2-dioleoyl-sn-glycero-3-phospho-L-serine; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DPPG, 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine polyethylene glycol; DSPG, 1,2-distearoyl-sn-glycero-3-phosphoglycerol; EPC, egg phosphatidylcholine; EPG, egg phosphatidylglycerol; HSPC, hydrogenated soybean phosphatidylcholine; QS-21, Quillaja saponaria Molina, fraction 21)
Emulsions
In an oil-in-water (o/w) emulsion, an oil phase is finely dispersed in an aqueous phase. Since this fine distribution is thermodynamically unstable, an amphiphilic emulsifier is needed to separate and stabilize the two phases (oil and water). Phospholipids are ideally suited for this purpose.
Egg phospholipids are being used as emulsifier in many o/w emulsions comprising various oils. They are used for parenteral nutrition (for example Intralipid® and Liposyn®) as 10 or 20 % o/w emulsions, up to a maximal cumulative daily dose of 3 g triglycerides/kg body weight/day, i.e. 0.36 g phospholipid/kg body weight/day.1)
Table 3 shows some examples of intravenous o/w emulsions using egg phospholipids as emulsifiers. O/w emulsions can also be used as carrier for oil-soluble drug substances. Also, in this case egg phospholipids are exclusively being applied as emulsifier. Table 4 provides some examples for intravenous, drug-containing o/w emulsions.
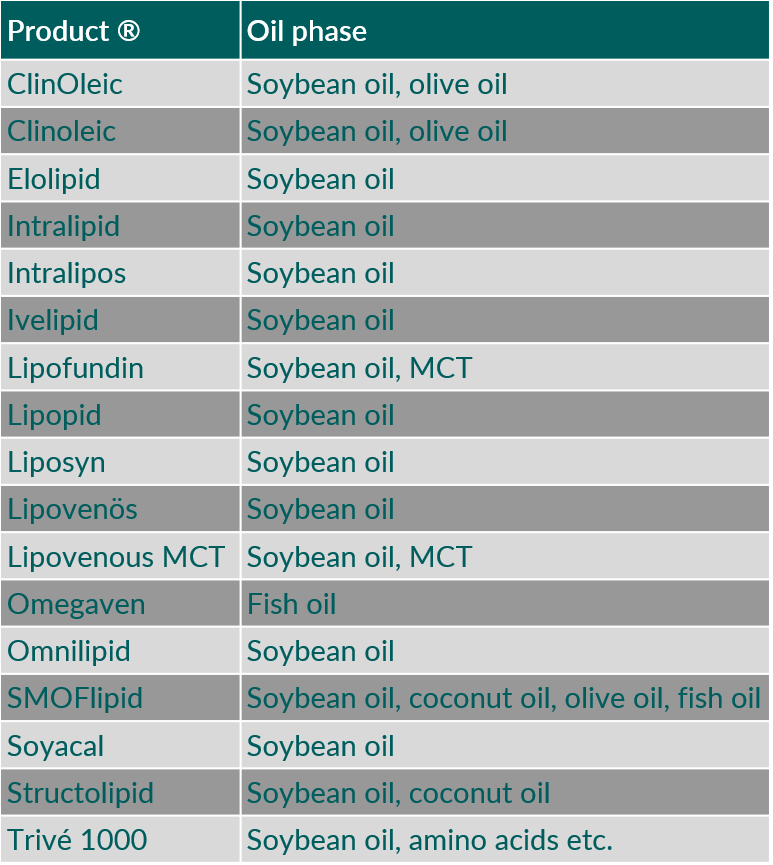
Table 3. Intravenous oil-in-water emulsions using egg phospholipids as emulsifier. (MCT, medium chain triglycerides)
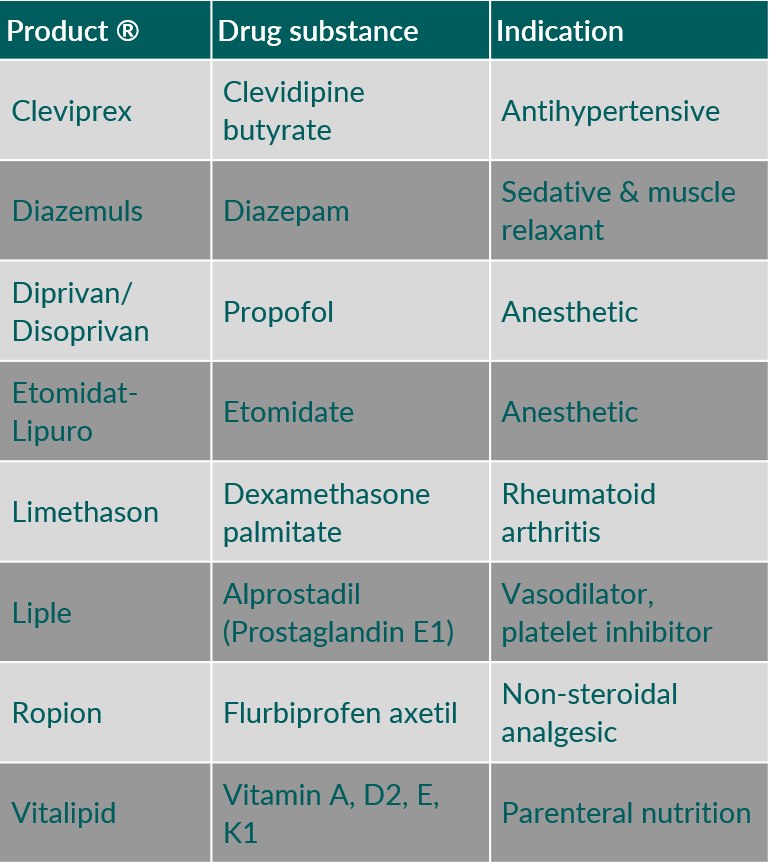
Table 4. Intravenous drug products containing oil-in-water emulsions using egg phospholipids as emulsifier.
Mixed Micelles

In mixed micellar formulations, comprising phospholipids and cholate salts, exclusively soybean phospholipids are being used. These formulations are either applied as solubilizer for poorly water soluble compounds, or the soybean phosphatidylcholine is intended as active principle, through the presence of poly-unsaturated fatty acids (PUFAs) for treatment of liver disorders.1)
Table 5 provides examples of intravenous mixed micellar products containing soybean phosphatidylcholine as phospholipid component.
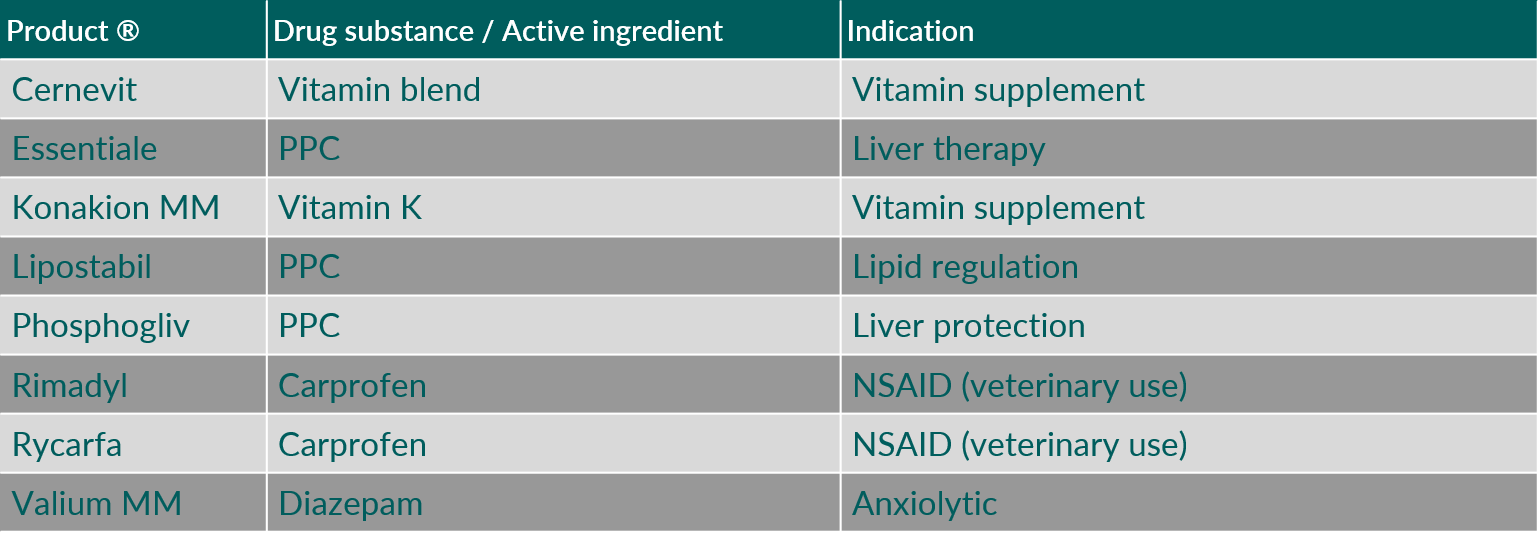
Table 5. Intravenous mixed micellar products containing soybean phosphatidylcholine as phospholipid component. (Abbreviations: NSAID, nonsteroidal anti-inflammatory drug; PPC, polyenyl-phosphatidylcholines)
Lipid Nanoparticles (LNPs)

Synthetic phospholipids can be found in vaccines, in the form of lipid nanoparticles (LNPs) or liposomes in recently developed vaccine formulations for intramuscular (i.m.) administration for the delivery of messenger ribonucleic acid (mRNA) and a disease-related antigen, respectively. LNPs are also used for intravenous (i.v.) administration of small interfering RNA (siRNA) in the treatment of rare diseases.
Table 6 gives some examples of parenteral products containing synthetic phospholipids used for vaccines and RNA delivery.
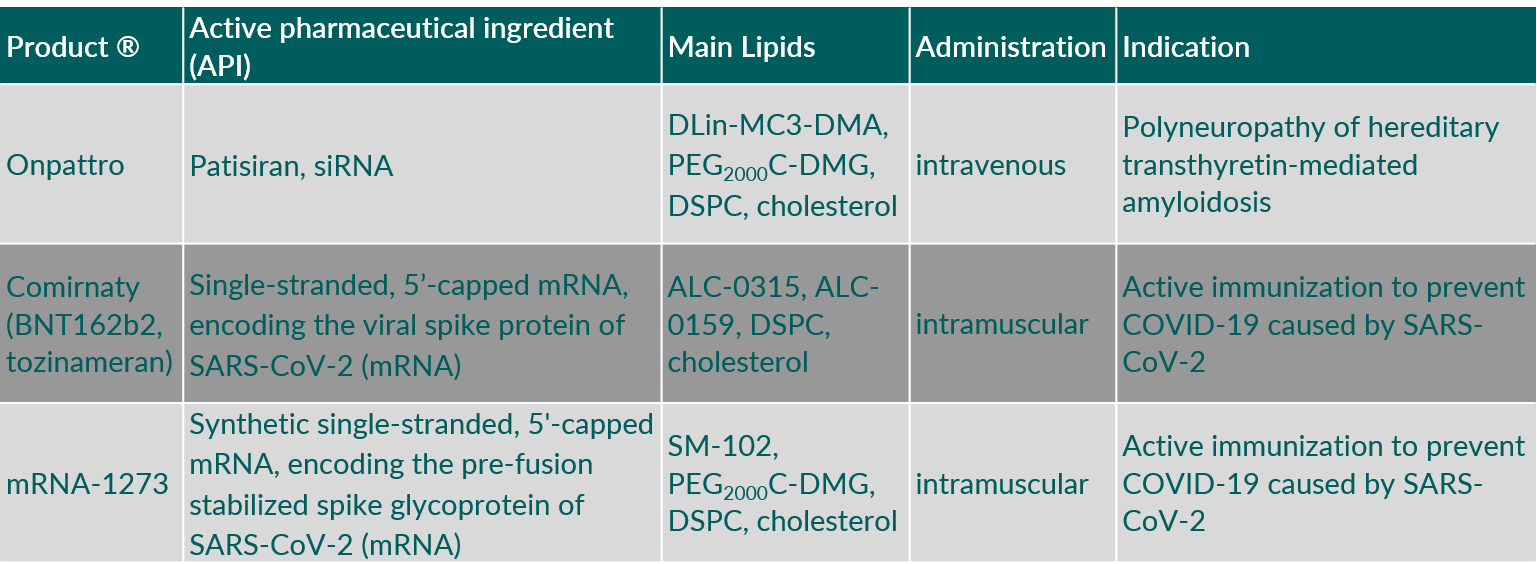
Table 6. Parenteral products containing synthetic phospholipids used for vaccines and RNA delivery. (Abbreviations: ALC-0159, 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide; ALC-0315, ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate); DLin-MC3-DMA, (6Z,9Z,28Z,31Z)-heptatriaconta-6,9,28,31-tetraen-19-yl-4-(dimethylamino)butanoate; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; PEG2000C-DMG, 3’-({[1,2-di(myristyloxy)propanoxy]carbonylamino}propyl)-ω-methoxy, polyoxyethylene.
You want to know more?
Please use the following links:
What phospholipid types are there?
What phospholipid aggregates are formed?
What is the difference between natural and synthetic phospholipids?
What is the natural occurrence and phospholipid benefit?
The use of natural and synthetic phospholipids as pharmaceutical excipients
Eur. J. Lipid Sci. Technol. 116, 1088-1107
| PubMed |
Role of phospholipids in the oral and parenteral delivery of poorly water soluble drugs
J. Drug Delivery Sci. Technol. 21, 5-16
| Elsevier |
Increasing drug solubility by means of bile salt-phosphatidylcholine-based mixed micelles
Eur. J. Pharm. Biopharm. 46, 361-367
| PubMed |
Bovine surfactant therapy for patients with acute respiratory distress syndrome
Am. J. Respir. Crit. Care Med. 155, 1309-1315
| PubMed |


