Lyso phosphatidylcholine for the stabilization of pharmaceutical proteins against adsorption and aggregation
Prof. Dr. Wolfgang Frieß1) – LMU, Munich, Germany
People involved
Ms. Eleni Papadopoulos (PhD fellow sponsored by the PRC) – Ludwig-Maximilians-Universitaet Muenchen, Germany (eleni.papadopoulos@cup.uni-muenchen.de)
Abstract
Biopharmaceuticals, and precisely protein drugs, have become the leading group of molecules in drug development and worldwide drug sales. They are almost exclusively formulated as aqueous solutions for injection, potentially after reconstitution of a lyophilisate. Unfortunately, proteins suffer from colloidal, conformational, and chemical instability. Surfactants are an almost essential part of the formulation to protect the protein molecules against damage induced at surfaces and by mechanical stress. The typically used polysorbates have several disadvantages: they (i) are mixtures of numerous different chemical species, (ii) may cause adverse effects, (iii) are instable themselves, and (iv) can trigger chemical degradation of proteins. Various critical issues with polysorbate in marketed products and development candidates have sparked the strive for alternatives.1)2)3)
Lyso-phosphatidylcholine (Lyso-PC) and related molecules as water soluble variants of lecithin show enormous potential with lecithin being approved for parenteral use. While Lyso-PCs are widely utilized for cosmetic, food and other pharmaceutical applications, they have not been tested with respect to their capacity to stabilized protein drugs. The marked hemolytic activity of Lyso-PCs, shown in literature, is indeed a concern. However, we are going to analyze this issue in more detail.
In this project, we will study the protection of monoclonal antibodies (MABs) and cytokines by Lyso-PCs for the first time in detail. Lyso-PCs of different purity, in hydrated and non-hydrated form, as chain pure variants and with variable headgroups will be considered. The key focus will be on the mechanistics and extent of surface protection of proteins considering air, silicone oil, tubing material, and ice.4) These studies are flanked by additional experiments on surfactant properties, hemolytic activity, stability, and direct interactions with protein molecules. Stress studies will be conducted, followed by mandatory long-term stability tests of different liquid protein formulations, thoroughly analyzing the stability of both the proteins and Lyso-PC itself. An additional important work package will focus on the protein stabilizing effect of Lyso-PC during freezing, freeze-drying, and upon storage.
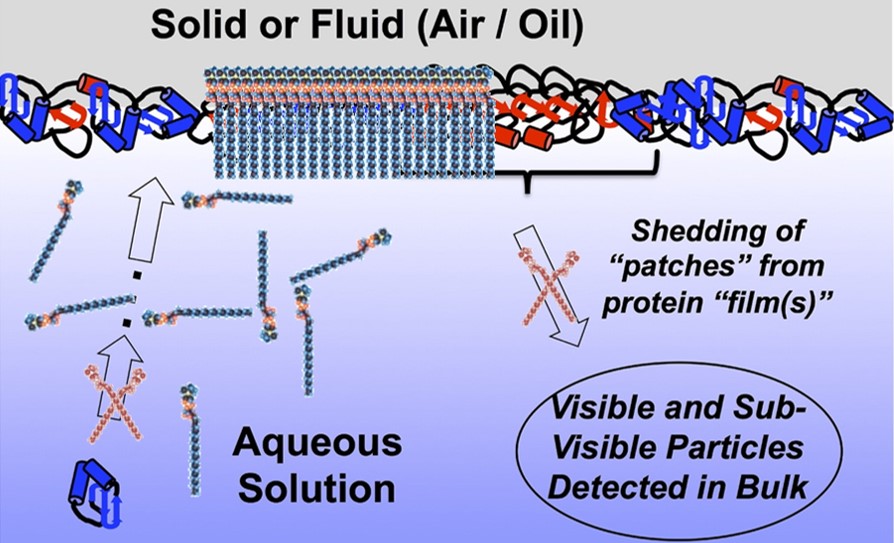
Figure 1. Schematic illustration of the project.
Benefit for the community
Stabilization of biologics is a highly important topic. With the sensitive biologics more and more taking over the lead in drug research, development and therapy including COVID-19 vaccines concerns of patient safety, e.g., when it comes to complex cellular and immune responses raise. The current state-of-the-art use of polysorbates has been challenged by many research studies. Alternatives are necessary which help to improve quality and safety of biologics. In addition, more stable biologics may benefit from storage at room temperature which brings a clear benefit in sustainability and transport in less developed regions of the world.
Visit the supervisors lab
Trends on Analytical Characterization of Polysorbates and Their Degradation Products in Biopharmaceutical Formulations
J. Pharm. Sci. 106, 1722-1735
| PubMed |
Synthesis, characterization and assessment of suitability of trehalose fatty acid esters as alternatives for polysorbates in protein formulation
Eur. J. Pharm. Biopharm. 76, 342-350
| PubMed |
The film tells the story: Physical-chemical characteristics of IgG at the liquid-air interface
Eur. J. Pharm. Biopharm. 119, 396-407
| PubMed |


