Research on the drug loading of exosomes
Prof. Dr. J.-C. Leroux1), ETH Zurich/Switzerland
Abstract
Stem cell therapy is often applied in the management of difficult-to-treat or yet incurable diseases, such as cardiac infarction, graft-versus-host and Alzheimer’s disease. With few exceptions, the application of living cells for therapeutic purposes has not yet fully found its way into standard clinical practice, primarily due to safety issues and an obscure mode of action. Instead, stem-cell derived exosomes have emerged as key mediators of stem cell biological effects, and additionally allow for elimination of the safety concerns associated with living cells. Furthermore, the inherent therapeutic efficacy of exosomes can be improved by encapsulation of appropriate drugs within their structure, thereby rendering them excellent candidates to substitute cell-based therapies in the future.
Although exosomes have been intensely exploited as drug delivery vehicles derived from natural sources, there is only limited access to efficient and mild drug loading procedures, especially for hydrophilic drugs.1)2)3)4)
The overall objective of this project is to engineer exosomes with enhanced anti-inflammatory activity. To achieve this goal, a robust method for the reproducible purification of functionally preserved stem cell exosomes will be optimized and fully validated. Subsequently, a loading method will be established allowing an efficient encapsulation of the anti-inflammatory hydrophilic model-drug pentoxifylline into these exosomes, while preserving their integrity. Therefore, a promising novel loading strategy based on the fusion of exosomes with drug-loaded liposomes will be investigated (Figure 1). Different fusogenic lipid classes and liposomal formulations thereof will be tested to optimize the fusion and, consequently, the drug loading efficiency. Eventually, the efficacy and integrity of the drug-loaded exosome-liposome fusion product will be analyzed in relevant in vitro experiments.
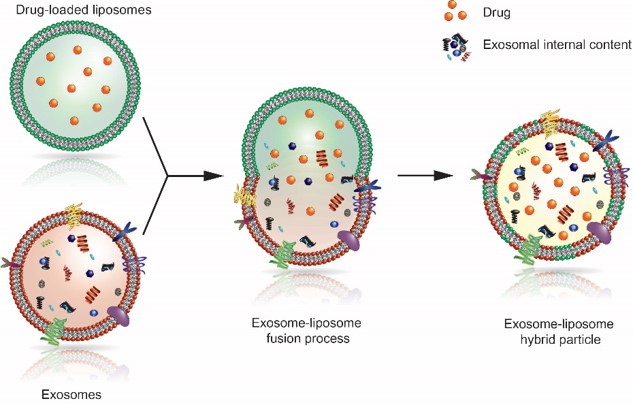
Figure 1. Schematic representation of the fusion process between exosomes and drug-loaded liposomes.
Benefit for the community
In recent years, exosomes have been the subject of an exponentially increasing number of high impact publications describing compelling evidence of their involvement in numerous biological processes. Furthermore, it is anticipated that these naturally-derived drug delivery vehicles may be pharmaceutically superior to several state-of-the-art synthetic drug carriers.
With this explorative and fundamental project, we aim at establishing a platform technology for the drug loading of exosomes. This will enable their application as a novel drug delivery strategy in a broad range of therapeutic indications. Moreover, should the fusion approach prove successful, it will not only be a facile method to encapsulate drugs, but also to simultaneously tailor the surface properties of the exosomes in a highly controlled fashion.
Visit the supervisors lab
Applying extracellular vesicles based therapeutics in clinical trials–an ISEV position paper
J. Extracell. Vesicles 4, 30087
| PubMed |
Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems
ACS Nano 12, 6830-6842
| PubMed |
Encapsulation of Hydrophilic Compounds in Small Extracellular Vesicles: Loading Capacity and Impact on Vesicle Functions
Adv. Healthc. Mater. 11, e2100047
| PubMed |


