Improving endosomal escape of lipid nanoparticles
Dr. Christoffer Åberg1) – University of Groningen/The Netherlands and Prof. Dr. Enrico Mastrobattista2) – Utrecht University/The Netherlands
1.
2.
People involved
M.Sc. Zhuqing Dai (PhD student funded by the PRC) - University of Groningen, The Netherlands
Abstract
Lipid nanoparticles (LNPs) have recently seen immense success for the delivery of messenger RNA (mRNA), both for therapy and vaccination. This success notwithstanding, there are several challenges holding back an even wider usage. While LNPs can readily escort the mRNA into cells, the clear majority of particles remains trapped in the endo/lysosomal compartments of the cell and the mRNA is thereby not delivered to the cytosol to exert its function.
This project aims to improve the efficiency of escape from the endosomes by a quantitative study of how LNPs enter, distribute within, and exit cells. We will create a library of LNPs with different properties using automatic microfluidic preparation. We will subsequently screen the LNPs for their cell uptake, endosomal escape and mRNA expression. Based on these observations, we will identify strategies on how to improve the efficiency of endosomal escape. We will test these strategies in more detailed studies where each step of the process is studied: cell internalization, endolysosomal processing, endosomal escape and potential cell export.
Ultimately, our strategies to achieve more efficient endosomal escape will support better mRNA delivery in future.
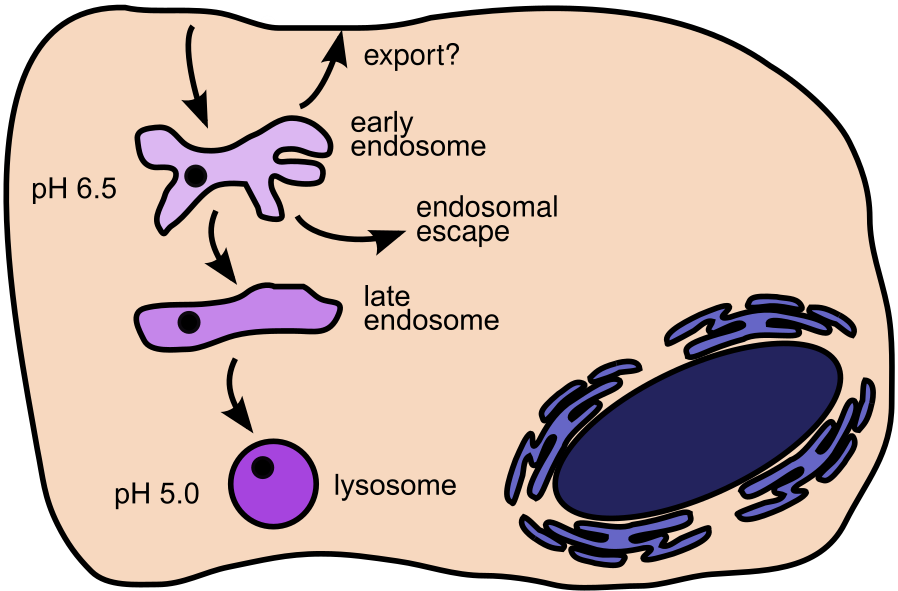
Figure 1. LNP internalization and subsequent endolysosomal processing (simplified). After internalization, the particle is delivered to an early endosome and subsequently reaches the late endosomes and, ultimately, the lysosomes. For mRNA delivery, escape from the endosomes is necessary. Note that the location where the particle/cargo escapes1)2)3) (here depicted as the early endosome) will be investigated in the project, as will potential export.
Benefit for the community
Escape from the endosomes is the perhaps most important bottleneck towards achieving efficient delivery of nucleic acids (siRNA, mRNA or DNA) or, indeed, macromolecules in general.4)5)6)7)
For lipid nanoparticles (LNPs) specifically it has been reported that only a few percent of internalized particles manages to release their cargo.1)2) Any improvement on this state of affairs is consequently going to have a large impact; for example, by increasing the endosomal escape of LNPs from 1 to 5%, a 5 times lower dose can be used, thereby reducing the occurrence of side effects, not to mention that 5 times more patients can be treated with the same production capacity. In times of a pandemic outbreak, this would make a huge difference. The outcome of this project will be a set of strategies for how best to achieve endosomal escape.
Overall, our intention is that anyone working on LNP-based delivery (and potentially drug delivery in general) can use our strategies and observations to improve the performance of their respective drug delivery platform in future.8)9)
Visit the supervisors lab
Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape
Nat. Biotechnol. 31, 638-646
| PubMed |
Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown
Nat. Biotechnol. 33, 870-876
| PubMed |
Endosomal escape of delivered mRNA from endosomal recycling tubules visualized at the nanoscale
J. Cell. Biol. 221, e202110137
| PubMed |
Challenges in carrier-mediated intracellular delivery: moving beyond endosomal barriers
Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 8, 465-478
| PubMed |
Intracellular delivery of nanomaterials: How to catch endosomal escape in the act
Nano Today 9, 344-364
| ScienceDirect |
Enhancing endosomal escape for intracellular delivery of macromolecular biologic therapeutics
Sci. Rep. 6, 32301
| PubMed |
Imaging of nanoparticle uptake and kinetics of intracellular trafficking in individual cells
Nanoscale 13, 10436-10446
| PubMed |
Impact of Formulation Conditions on Lipid Nanoparticle Characteristics and Functional Delivery of CRISPR RNP for Gene Knock-Out and Correction
Pharmaceutics 14, 213
| PubMed |


