Receptor-targeted lipid nanoparticles for mRNA delivery into T lymphocytes
Prof. Dr. Christian Buchholz1) – Paul-Ehrlich-Institut/Germany
People involved
(to be added later)
Abstract
RNA-delivering lipid nanoparticles (LNPs) have become key for vaccine development during the coronavirus pandemic. Beyond vaccination, delivery of mRNA encoding therapeutic proteins by LNPs is establishing as alternative to viral vectors in gene therapy.1) However, the clear majority of therapeutic strategies using RNA-LNPs focus on liver as target tissue, since the biomolecular corona formed on LNPs in vivo mediates a strong liver tropism. Based on current understanding, this is due to apolipoprotein E (ApoE) which binds LNPs as well as low-density lipoprotein receptor (LDLR), which is highly expressed in liver.
This project aims at generating receptor-targeted RNA-LNPs that exhibit an extraordinarily high target cell selectivity while avoiding delivery to off-targets such as liver. To achieve this, the applicant will transfer a strategy he successfully developed for viral gene transfer vectors to LNPs.2) A high-affinity ligand for the target receptor of choice, in this case CD8, will be displayed on the LNP surface. As CD8 binders, designed ankyrin repeat proteins (DARPins) directed against human or mouse CD8 will be incorporated into LNPs. Binding of the particles as well as RNA delivery to CD8+ T cells will be monitored in comparison to conventional LNPs on T cell lines and primary donor lymphocytes isolated from blood. In vivo RNA delivery and biodistribution will be assessed in mouse models.
This work will result in basic insights into the biodistribution of RNA-LNPs as well as novel tools for mRNA delivery in cancer immunotherapy. Beyond that, the strategy for receptor-targeting of LNPs can be easily adapted to any cell surface receptor of choice, thus further expanding potential applications of phospholipid research into various aspects of in vivo gene therapy.
Benefit for the community
RNA delivery to tissues other than the intended target cells, as it occurs with current LNP technology, can be detrimental and risky. Moreover, from an economic standpoint, off-target delivery reduces the number of particles available for the therapy-relevant cells. This increases the required dose to compensate for imperfect targeting, thus raising not only costs for the therapy, but also the risk for serious adverse effects. So far, non-viral vector tools that would mediate precise delivery to the therapy-relevant cell type do not exist. The outcomes of this proposal will exactly address this issue by establishing a novel approach for LNP engineering resulting in CD8-targeted RNA-LNPs. The approach is designed such that it can be easily adapted to any other cell type or target receptor beyond CD8, simply by exchanging the DARPin displayed on the LNP surface. Compared to antibodies, we expect from DARPins an increased coupling efficiency due to the easy introduction of a functionalizeable thiol group via a cysteine at their C-terminus. Since DARPins lack cysteine, side products due to the coupling chemistry are highly unlikely. Moreover, DARPins are much smaller, and, due to a α-helical structure lacking any disulfide bonds, much more stable than conventional antibodies. As demonstrated for viral vectors, we expect that DARPins will also increase on-target delivery of LNPs.
The implications of this project will be highly relevant for the whole field of vector development and gene therapy. The CD8-targeted LNPs can have major implications for CAR T cell therapy. Currently, CAR T cells are an individualized medicinal product (Figure 1A). This process entails not only high production costs but also subjects the T cells to various modifications, thereby altering their phenotypes and activities. Both issues limit the applicability of this therapeutic concept to the mass-market of cancer patients. The direct in vivo transfer of CARs by CD8-LNPs could convert the autologous cell-based products to a universally applicable off-the-shelf drug (Figure 1B). While this will need further preclinical studies going beyond the scope of this proposal, this scenario is well in reach, since it will only require the packaging of the CAR encoding mRNA instead of a reporter.
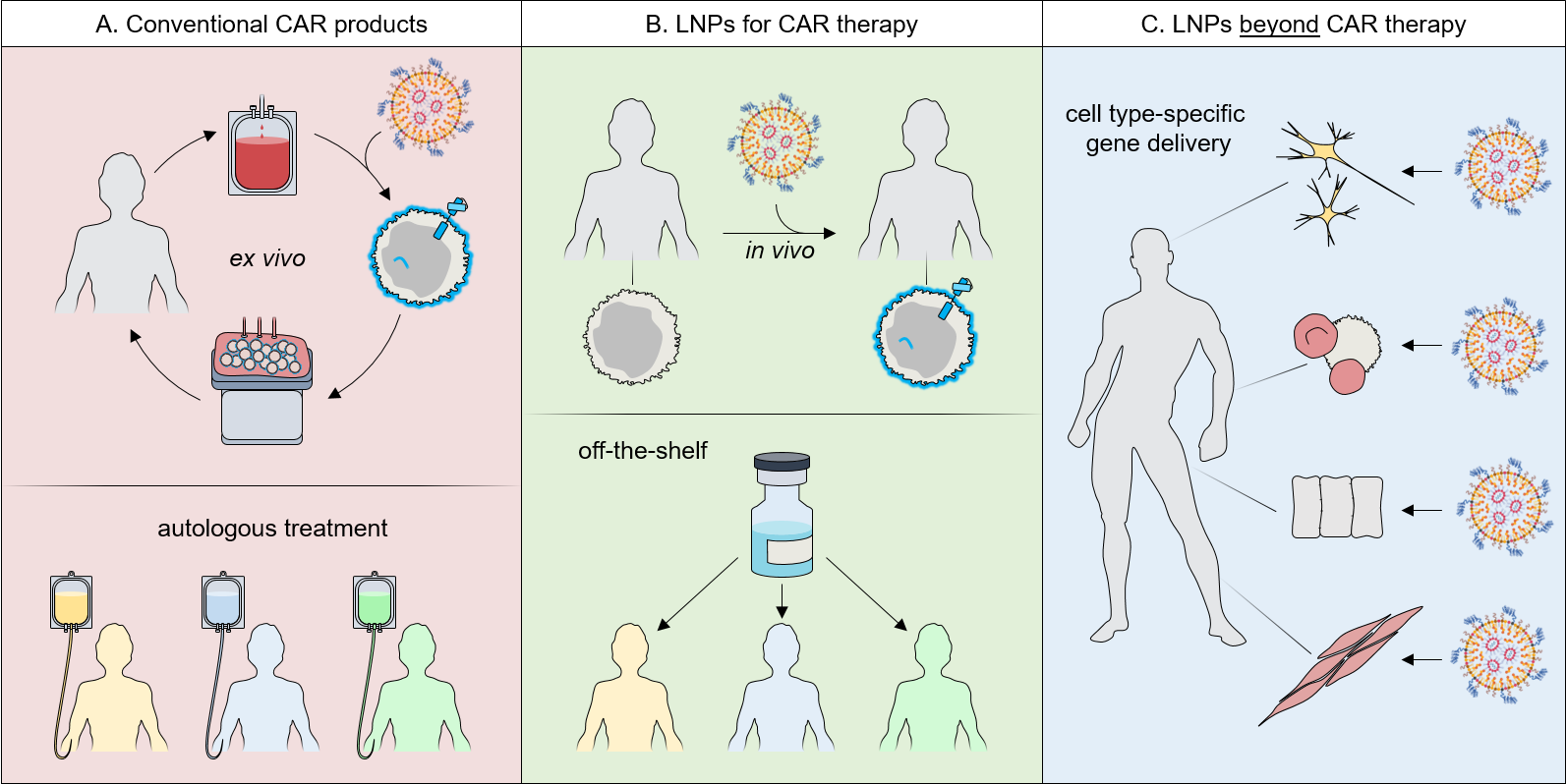
Figure 1. Impact of CD8-LNPs for CAR T cell therapy and beyond: Comparison of conventional (A) and in vivo CAR T cell generation (B). The genetic information for the CAR (blue) is delivered into the patients’ T lymphocytes. The current conventional approach results in an individualized medicinal product for each patient, while in vivo delivery results in an off-the-shelf product. Beyond CAR T cells, receptor-targeted LNPs will open many novel therapeutic strategies involving various therapy-relevant cell types (C).
Since not only the therapeutic mRNA can be easily exchanged but also the DARPin and thus target cell selectivity, the LNP platform developed in this proposal can enable various novel therapeutic strategies (Figure 1C). Target cells can be part of the hematopoietic system such as NK cells, a rising alternative in cellular immunotherapies, B cells for in vivo antibody production or hematopoietic stem cells, which are the main target for curing monogenetic diseases of the hematopoietic system. Other tissues are of equal relevance. Targeting particular cell types of the brain or of the heart are only two examples of a long list of opportunities. In that context, it is important to mention that LNPs can also be used to induce stable genomic modifications when delivering guide RNA together with mRNA encoding endonucleases such as Cas9. All these potential strategies, once established, will substantially reduce costs and make certain diseases, for which therapy-relevant cells cannot readily be explanted, cultivated and reintroduced, treatable for the first time
Visit the supervisors lab
Prof. Dr. Christian Buchholz


