Radiation-responsive liposomes for controlled release and tumor permeation of radiotherapy dose enhancers
Dr. M. Broekgaarden1), University Grenoble, Institute for Advanced Biosciences, La Tronche, France
People involved
Dr Mans Broekgaarden (Postdoc sponsored by the PRC) - Institute for Advanced Biosciences (mans.broekgaarden@univ-grenoble-alpes.fr)
Abstract
To improve the management of treatment-resistant cancers, there is a continued need for new strategies to augment radiotherapy outcomes. The use of ultra-small gold particles (nanoclusters) is a promising way to improve radiation absorption by cancer tissues, yet their therapeutic potential is limited because of poor tumor accumulation and permeation.1)2) This project aims to engineer an innovative liposomal modality to overcome these limitations, thereby enabling the gold nanoclusters to hyper-sensitize cancer tissues to radiotherapy.3)
First, liposomes containing oxidation-susceptible phospholipids will be engineered to contain gold nanoclusters in their aqueous core and novel phospholipid-anchored photosensitizers in their lipid bilayer. During photodynamic therapy or radiotherapy, these compounds will generate high levels of reactive oxygen species that will oxidize the liposomal lipids and release the gold nanoclusters in the tumor microenvironment (Fig. 1). 4)5)6)
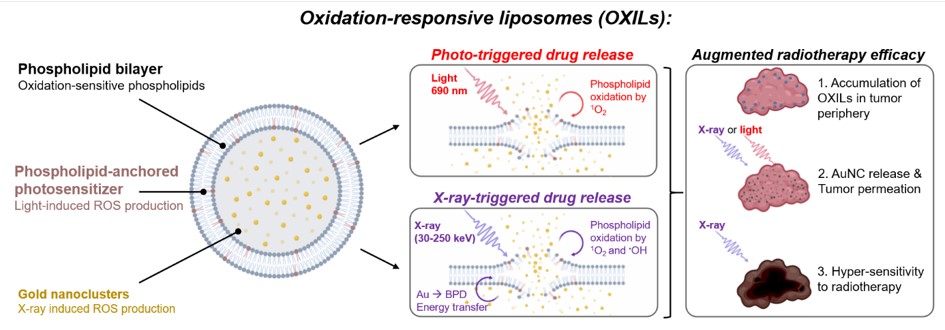
Figure 1. Schematic overview of the oxidation-responsive liposomes and the light- and X-ray-induced drug release mechanisms that will be developed in this project.
The cytotoxicity of the oxidants will also alleviate the high cell densities in the tumor periphery, enabling the gold nanoclusters to deeply permeate and effectively radiosensitize the cancer tissues. To develop this modality, radiation-triggered nanocluster release from liposomes will be studied using monochromatic synchrotron radiation, whereas tumor permeability and radiotherapy efficacies will be studied using state-of-the-art microtumor cultures combined with adaptive optics microscopy. The project is expected to provide compelling proof-of-concept for light- and X-ray-controlled liposomal drug release that will warrant further in vivo investigations. Combining phospholipid-anchored photosensitizers with heavy metal nanoclusters to achieve radiotherapy-controlled drug release from liposomes is a ground-breaking novel concept that will be developed during this project. Moreover, the capacity of this approach to permeabilize tumor tissues to cancer therapeutics has never been demonstrated.
Benefit for the community
New strategies to treat cancer are in constant demand as global patient numbers continue to increase. After surgery, radiotherapy remains one of the most frequently used cancer treatments, while photodynamic therapy is emerging as a promising alternative for terminal and treatment-resistant cancer types. The innovative liposomal drug delivery modality developed in this project can be applied to both photodynamic- and radiotherapy, is unique in its capacity to simultaneously control drug release and permeabilize cancer tissues and is customizable for the accommodation of alternative cancer therapeutics. The OXIL modality thus carries the potential to augment various clinically relevant therapies that can benefit the treatment of a broad spectrum of cancer types.
Taken together, this project will provide exciting new avenues for light- and radiation controlled liposomal drug delivery that can significantly advance the design of new liposomal pharmaceuticals.
Visit the supervisors lab
Development and in vitro proof-of-concept of interstitially targeted zinc- phthalocyanine liposomes for photodynamic therapy
Curr. Med. Chem. 21, 377–391
| PubMed |
Surface functionalization of gold nanoclusters with arginine: A trade-off between microtumor uptake and radiotherapy enhancement
Nanoscale 12, 6959-6963
| PubMed |
Modulation of redox metabolism negates cancer-associated fibroblasts-induced treatment resistance in a heterotypic 3D culture platform of pancreatic cancer
Biomaterials 222, 119421
| PubMed |
Impacting pancreatic cancer therapy in heterotypic in vitro organoids and in vivo tumors with specificity-tuned, NIR-activable photo-immunonanoconjugates: Towards conquering desmoplasia?
Nano Lett. 19, 7573-7587
| PubMed |
Radiation dose-enhancement is a potent radiotherapeutic effect of rare-earth composite nanoscintillators in preclinical models of glioblastoma
Adv. Science 7, 2001675.
| PubMed |
Optimizing the Pharmacological and Optical Dosimetry for Photodynamic Therapy with Methylene Blue and Nanoliposomal Benzoporphyrin on Pancreatic Cancer Spheroids
Onco 2, 19-33.
| MDPI |
Generating Large Numbers of Pancreatic Microtumors on Alginate-Gelatin Hydrogels for Quantitative Imaging of Tumor Growth and Photodynamic Therapy Optimization
Methods Mol. Biol. 2451, 91-105.
| PubMed |


