Phospholipid Microemulsions for the Dermal Application of Proteins: Prediction of in-vivo Skin Tolerability with Full Thickness Human Skin Equivalent
Prof. Dr. R. Scherließ1), Kiel University/Germany
Abstract
The development of a dermal formulation containing a protein drug offers novel therapeutic options, but also comprises new challenges regarding the formulation development. Aspects to consider are the high molecular weight of proteins, the polarity and their sensitivity to external conditions which most often result in a short product shelf-life. Hydrolytic sensitivity can be one critical property of a protein that causes a decreased stability and hence has been examined exemplary for the family of collagenases in this study. To ensure the successful development of a vehicle system for the dermal delivery it must be tolerated well on human skin. Microemulsions (MEs) or more precisely pre-microemulsion concentrates were chosen as promising topical formulations for hydrolytic sensitive proteins. Due to their spontaneous formation after mixing the ingredients, MEs enable a separated storage of the aqueous and the lipophilic phase in a two-chamber-system. This special composition isutilised for the protein stability as the protein is suspended in the lipophilic phase during storage and only dissolved in the aqueous part of the ME shortly before the application. Therefore, the contact time between protein and water is reduced to a minimum. For the ME formation Lipoid®S LPC 80 (Lip80) was chosen as surfactant due to its high self-emulsifying power besides the enhanced skin penetration effects and good tolerability. Formulation stability and the ability to serve as protein vehicle system were analysed with a model enzyme in comparison to a reference ME stabilised by PEGylated surfactants. Since proteins are high molecular weight drugs, a microneedle device supported the penetration to overcome the skin barrier. Dermastamp® was selected and applied in combination with Lip80-ME as well as with the reference ME in tolerability studies with a 3D-full-thickness skin model. The immunological response was measured by the release of cytokines (IL-1α, IL-6, IL-8), the cell viability (LDH) and morphological changes. Microneedling is a minimal invasive procedure, stimulating a local irritation. Thus, the need for mild and well-tolerated formulations iseven increased.
The stability and activity of model protein in Lip80-ME were more favourable than the reference ME. The superiority of Lip80-ME was confirmed by a lower irritation potential on reconstructed human skin. Moreover, this formulation showed the property to reduce the local irritation caused by the needle device. To sum up, the developed combination of Dermastamp® and Lip80-ME is a promising approach in the dermal application of hydrolytically sensitive proteins.
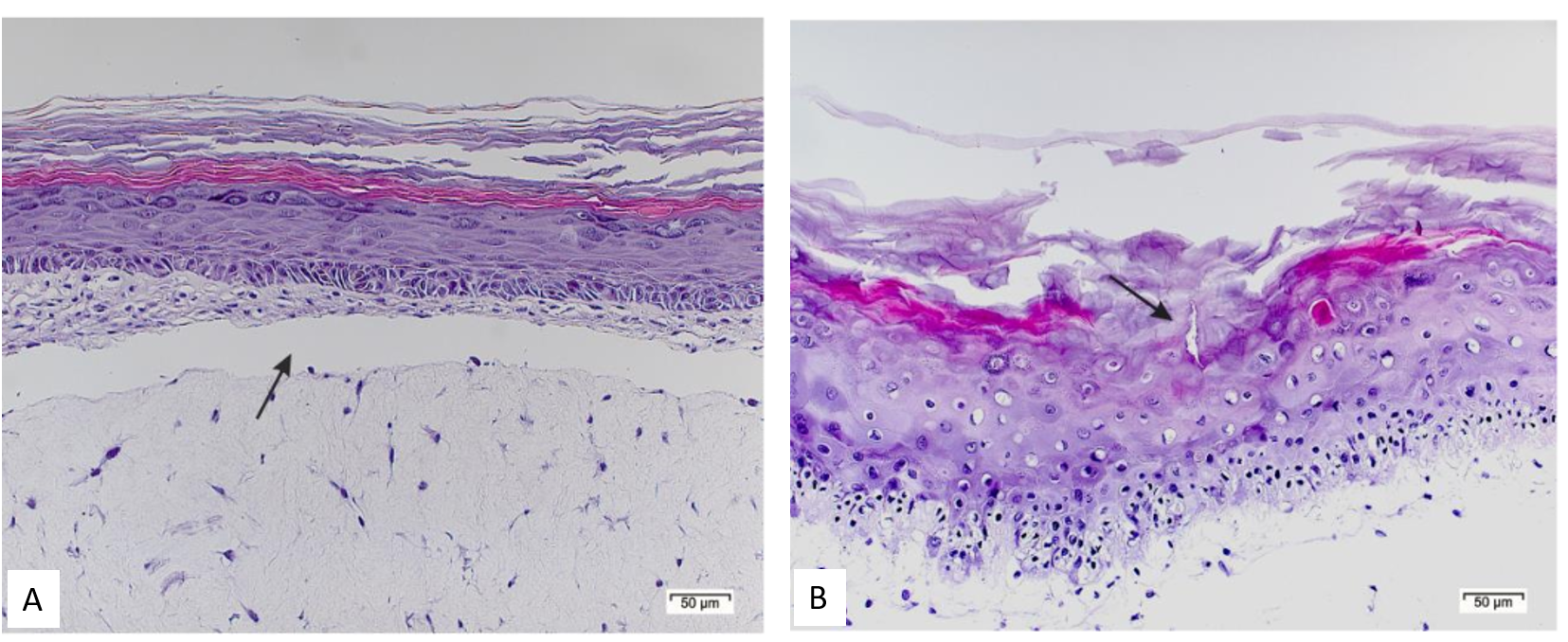
The image shows histopathological examinations of EpiDermTM FT skin model after 8 h exposure of (A) Dermastamp® applied with Lip80-ME and (B) Dermastamp® applied with reference ME based on PEGylated surfactants. 1)
Visit the supervisors lab
Dermal Application of Proteins: Development and Characterization of an Application System
PhD Thesis, Kiel University
Evaluation of Microneedle Devices for the Application of Dermal Protein Formulations
4th Galenus Workshop "Drug Delivery to Human Skin"
| Google Scholar |


