On-demand amplification of chemotherapy by ultra-fast drug release from plasmonic liposomes
Dr. Xiuying Li1), University of Dallas/USA
People involved
Dr. Xiuying Li (Postdoc fellow sponsored by the PRC) – UTD, USA (xiuying.li@utdallas.edu)
Abstract
Multidrug resistance (MDR) is a major obstacle for successful cancer chemotherapy, and resistance to therapy has been correlated with the presence of efflux “pumps” to actively expel chemotherapy drugs from tumor cells. MDR is further enhanced by the endo-lysosomal drug sequestration. To amplify chemotherapy and overcome cancer MDR, new strategies are needed to escape the endo-lysosomal trap and rapidly release chemotherapy drugs inside tumor cells.1)2)
Herein we propose a novel intracellular ultra-fast drug release method based on plasmonic liposomes. Plasmonic liposomes is a new class of liposomes with a plasmonic gold coating and combines the superior advantages of liposomes and plasmonic nanoparticles. When activated by a tissue-penetrating near-infrared (NIR) laser pulse, transient plasmonic nanobubbles (PNBs) is generated to create a mechanical force and eject drug molecules out of liposomes, leading to instantly high intra-cellular drug concentration. This mechanical force also pushes drug molecules across endosome membrane into cytoplasm, circumventing the endo-lysosomal drug sequestration.3)4)5)
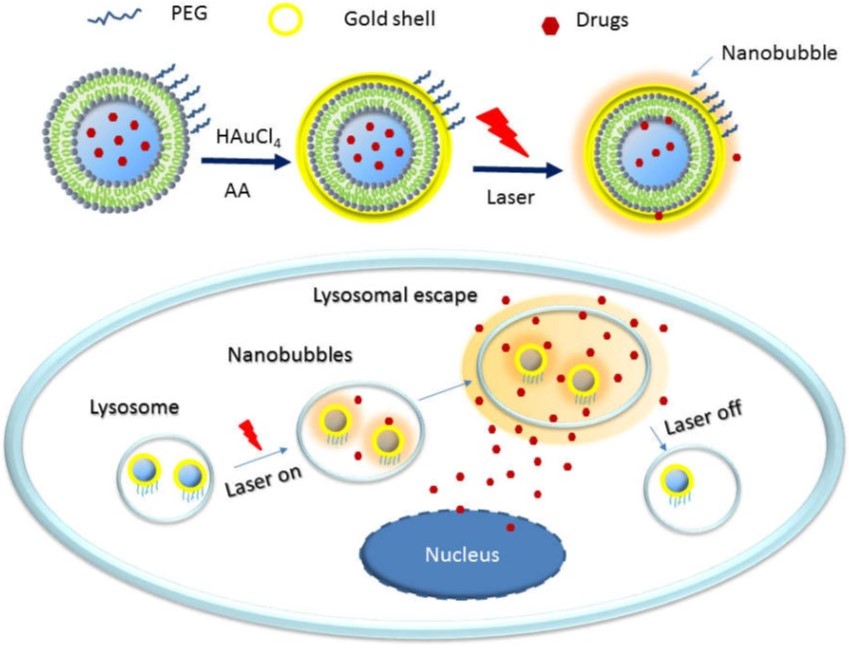
Figure 1 Illustration of intracellular fate of a drug loaded plasmonic liposome
The main aim of this study is to develop plasmonic liposomes with high PNBs yield efficiency to significantly enhance endo-lysosomal permeability and on-demand burst release of liposome cargos in tumor cells. Plasmonic liposomes composed of different phospholipids will be developed. The effects of phospholipids (natural versus synthetic, phospholipids with different phase transition temperatures, and with negatively or positively charged headgroup) on the interaction between liposome and gold coating and PNBs yielding efficiency will be investigated. Mechanisms to overcome the MDR to enhance the anti-tumor effect will be investigated in vitro and in vivo.
The outcome of this study will prove the feasibility of using plasmonic liposomes as a novel platform to amply chemotherapy and overcome MDR by ultra-fast intracellular drug release and enhanced endo-lysosomal escape. This study also will provide new knowledge on the interface of phospholipids with plasmonic nanomaterials and expand the application of liposome as plasmonic multifunctional nano-carriers.
Benefit for the community
This work focuses on the scientific and clinical challenge of multidrug resistance and poor drug control release in cancer cells. Plasmonic liposomes have been proposed and the ultra-fast drug burst release will be studied in vitro and in vivo. This work has several benefits for the scientific community. Firstly, it is helpful for further improvement of liposome-based drug delivery systems for their clinical application. Secondly, this strategy to enhance endo-lysosomal escape is also useful for other therapeutic approaches, including gene therapy, probing cell signaling, and neuromodulation.
Results/Outcome
In conclusion, we developed a NIR light-triggered ultrafast burst release technique, characterized its cargo release feature, and investigated its potential applications. This technique has several advantages, such as ultrafast burst release speed, high spatiotemporal resolution, and enhanced cargos nuclear entry upon cytosol burst release. We did some preliminary tests on its potential applications, including enhancing chemotherapeutic effect by enhanced intracellular release and nuclear accumulation of doxorubicin, modulation of cell calcium signaling by burst release of endogenous second messenger, enhancing gene transfection efficiency and the capability for high spatiotemporal in vivo burst release. Although some optimizations may be needed, this technique showed its potential in many different biological fields, for example macromolecule delivery to the cell nucleus for gene therapy or anticancer drug delivery.
Visit the supervisors lab
Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance
Oncotarget. 6, 1143-1156
| PubMed |
Combating apoptosis and multidrug resistant cancers by targeting lysosomes
Cancer Lett. 332, 265-274
| PubMed |
Cell-specific transmembrane injection of molecular cargo with gold nanoparticle-generated transient plasmonic nanobubbles
Biomaterials 33, 5441-5450
| PubMed |
Thermophysical and biological responses of gold nanoparticle laser heating
Chem. Soc. Rev. 41, 1191-1217
| PubMed |
Rock the Nucleus: Significantly Enhanced Nuclear Membrane Permeability and Gene Transfection by Plasmonic Nanobubble Induced Nanomechanical Transduction
Chem. Commun. 54, 2479-2482
| PubMed |
Ultrafast Near-Infrared Light-Triggered Intracellular Uncaging to Probe Cell Signaling
Adv. Funct. Mater. 27, 1605778
| PubMed |


