Liposomal oral drug delivery: The use of bipolar amphiphiles to stabilize liposomes
PD Dr. S. Drescher1), University Halle/Germany.
People involved
Sindy Müller, Dipl.Pharm. (PhD fellow sponsored by the PRC) - University of Halle, Germany
Abstract
Liposomes are one of the most successful drug delivery systems, not least because of the excellent entrapment capacity, biocompatibility, and safety. After Doxil® has become the first liposomal formulation receiving FDA approval in 1995,1) numerous liposomal formulations have been developed till the present. Despite the success of liposomes administered parenterally, the delivery of liposomes via the oral route is impeded by various barriers, namely the chemical instability of the components of the liposomes (phospholipids) in the gastrointestinal tract (GIT), the loss of integrity of the liposomes due to mechanical instability of the vesicles itself, and the poor permeability of conventional liposomes due to their relatively large size and the presence of various epithelial barriers in the intestine.
A very promising approach to improve the chemical and mechanical stability of liposomes in the GIT is the replacement of the classical monopolar phospholipids by bipolar amphiphiles, so-called bolalipids or bolaamphiphiles.2) These bolalipids can be inserted in a stretched manner in a phospholipid bilayer and can therefore act as a rivet to stabilize the bilayer and, hence, the liposome.
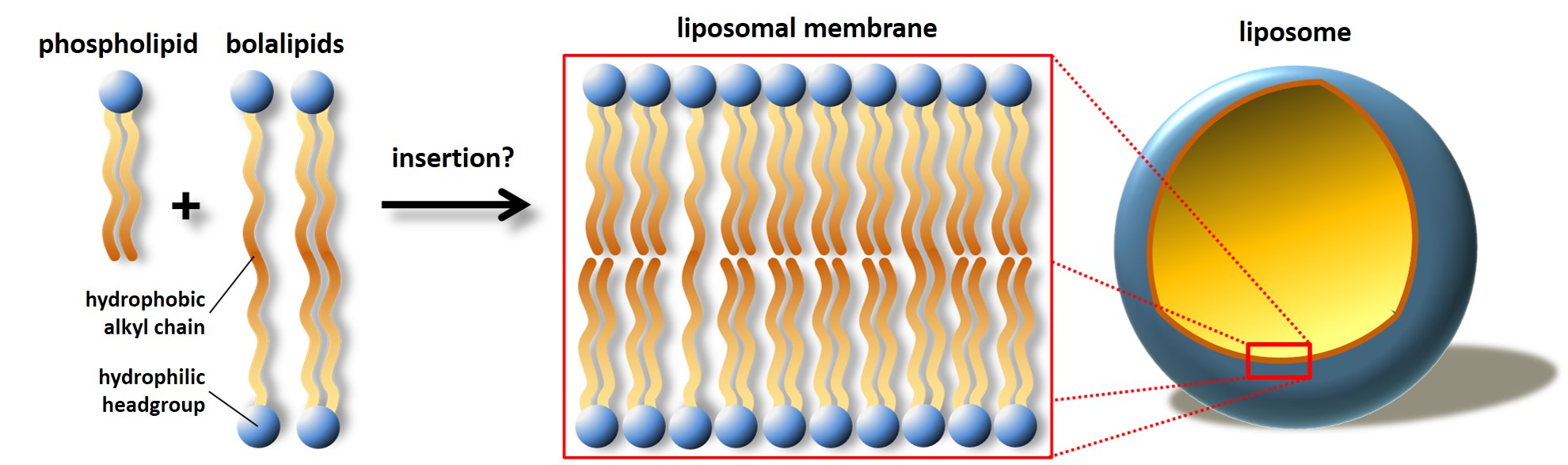
Figure 1. Schematic representation of a liposomal membrane containing classical monopolar phospholipids and bipolar bolalipids in a stretched manner. This insertion could lead to a stabilization of the liposome applicable for oral drug delivery.
Bolalipids are molecules that consists of a long alkyl chain and two hydrophilic headgroups attached to each end. This special class of lipid molecules can be found in the membranes of certain species of Archaea, where they contribute to the outstanding stability of the Archaea against harsh living conditions, such as low pH values, high salt concentrations, and/or high temperatures. However, the use of the natural occurring archaeal lipids is challenging due to the high costs of the cultivation of the Archaea. To circumvent these drawbacks, the group of Treusch try to establish alternative sources of natural bolalipids. On the other hand, also the total synthesis of these archaeal lipids is time-consuming, expensive, and in most cases only a small amount of product can be obtained.
Hence, the great vision of our group is to find an easy-to-synthesize bolalipid that can be used for the stabilization of orally administered liposomes.
Benefit for the community
In future, the development of a liposomal formulation stabilized by bolalipids could make the oral application of so far intravenously or subcutaneously administered drugs possible. This would reduce infection rates, side effects of highly potential drugs, and enhance the life quality of patients through a simple, self-administered application. Furthermore, the simplification of the chemical structure of artificial bolalipids can facilitate industrial production and keep the costs of production low. In addition, a well-defined substance is obtained compared to extracted lipids, which facilitates further processing.
Results/Outcome
An asymmetrical glycerol diether bolalipid with protonable phosphodimethylethanolamine headgroup: The impact of pH on aggregation behavior and miscibility with DPPC
Polymers 9, 573
| PubMed |
Impact of headgroup asymmetry and protonation state on the aggregation behavior of a new type of glycerol diether bolalipid
Langmuir 34, 4360-4373
| PubMed |
Mixing behaviour of asymmetrical glycerol diether bolalipids with saturated and unsaturated phosphatidylcholines
Biophys. Chem. 238, 39-48
| PubMed |
Synthesis and aggregation behaviour of single-chain, 1,32-alkyl branched bis(phosphocholines): Effect of lateral chain length
Org. Biomol. Chem. 16, 2711-2724
| PubMed |
Mixing behaviour of bi-layer-forming phosphatidylcholines with single-chain alkyl-branched bolalipids: Effect of lateral chain length
Biophys. Chem. 244, 1-10
| PubMed |
Bolalipid-Doped Liposomes: Can Bolalipids Increase the Integrity of Liposomes Exposed to Gastrointestinal Fluids?
Pharmaceutics 11, 646
| PubMed |
Synthesis and aggregation behaviour of single-chain, 1,32-alkyl-branched bis(phosphocholines) – part 2: lateral chain length triggers self-assembling from sheets to fibres to vesicles
Org. Biomol. Chem. 18, 3585-3598
| PubMed |


