Evaluation of cochleates as parenteral depot formulations
Assoc. Prof. Dr. J. Kuntsche1), University of Southern Denmark/Odense/Denmark
People involved
Søren Kristensen (PhD fellow sponsored by PRC) – SDU, Odense, Denmark
Abstract
The aim of the project is to explore cochleates as parenteral depot formulation for poorly water-soluble, lipophilic drugs. Moreover, the applicability to use negatively charged phospholipids that are phosphatidylserine (PS), phosphatidylglycerol (PG), and phosphatidic acid (PA) from natural sources such as purified soy-phosphatidylserine and reproducibility of the preparation process will be studied.
Cochleates are composed of tightly packed lipid bilayers formed by, e.g., PS in the presence of calcium ions.1) Due to the tightly packed lipid structure, cochleates generally provide improved protection against degradation and delayed release of entrapped (“encochleated”) drugs. Moreover, as cochleate formation is a reversible process, cochleates generally may provide the possibility of triggered drug release. Although cochleate formulations have already been studied for drug delivery purposes in some detail, their formation process as well as parameters influencing drug release are still not fully explored.
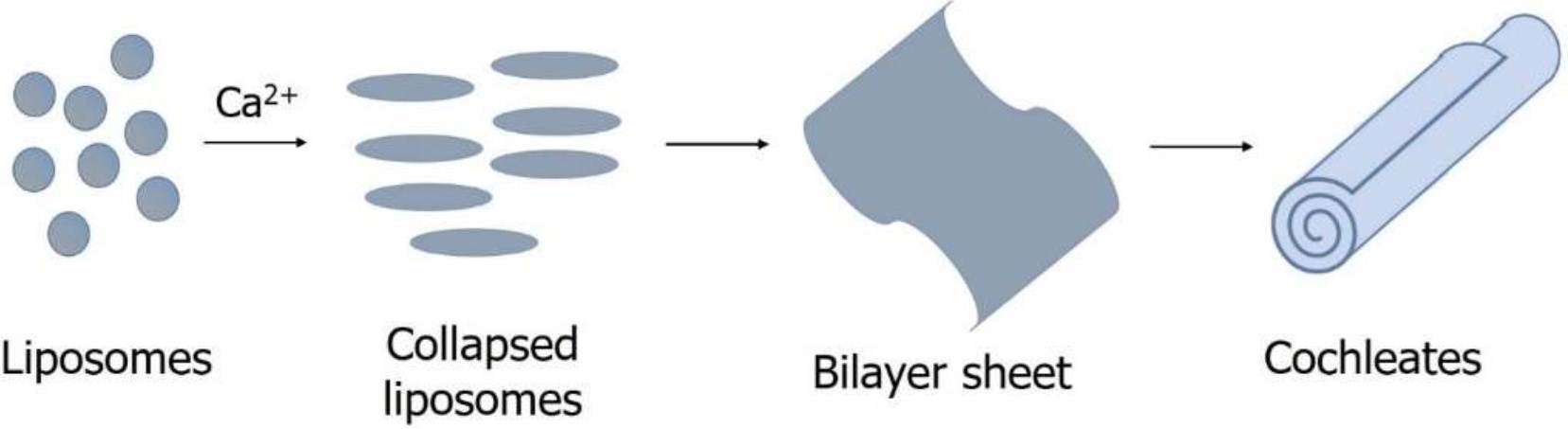
Figure 1. Proposed mechanism of cochleate formation (adapted from 1)).
The major new aspects addressed in this proposal are:
- the development and evaluation of cochleates as parenteral depot formulations (drug incorporation, in vitro drug release and stability, local toxicity and evaluation concerning drug plasma profile and potential fibrous encapsulation of the cochleates in vivo after s.c. administration),
- the use of negatively charged phospholipids from natural sources as a basis for reasonable and cost-effective development of drug formulations, and
- the evaluation of the production process concerning robustness, reproducibility and scaling up as a basis for industrial cochleate production.
Benefit for the community
The results of the project will contribute to a better understanding of the major parameters of cochleate formation and provide the basis for potential further industrial development of cochleate formulations for drug delivery, specifically as parenteral depot formulation.
Results/Outcome
The major aim of the project – to explore cochleate formulations based on phosphatidylserine (PS) from natural sources as parenteral sustained release formulation – has been achieved. Especially the results obtained with the soy PS and initial investigations on the influence of lipid concentration on DOPS aggregate structure are of high novelty.
Visit the supervisors lab
Cochleate lipid cylinders: Formation by fusion of unilamellar lipid vesicles
Biochim. Biophys. Acta 394, 483-491
| PubMed |
Feasibility of the preparation of cochleate suspensions from naturally derived phosphatidylserines
Front. Med. Technol. 5, 1241368.
| PubMed |


