Phospholipids for Injectable Depot Formulations
Richard Wibel and Peter Hölig, Lipoid GmbH, Frigenstr. 4, D-67065 Ludwigshafen, Germany (email: p.hoelig@lipoid.com)
Because many active pharmaceutical ingredients (APIs) have a short biological half-life, multiple administrations are needed to achieve a therapeutic effect. In addition, many APIs may not be suitable for oral administration because of degradation in the gastrointestinal tract (GIT) and poor absorption, including high molecular weight proteins and peptides.1)
For such drug substances, extended-release depot injectables for local or systemic applications enable a more patient-friendly, low frequency therapy, leading to a better adherence. Extended drug release can be achieved by various formulation strategies. Established formulation options include drug substance suspensions and polymer depots. Suspensions cover short release periods of hours to days, while long release periods of up to months can be achieved with polymer depots. For intermediate release periods, i.e. days up to several weeks, lipid- and especially phospholipid-based formulations are an interesting and innovative depot-injectable approach.2)
Phospholipids are natural components of every human cell and are therefore highly tissue-friendly and suitable for any local administration route, including subcutaneous and intramuscular administration. Due to the amphiphilic character of phospholipids, both lipophilic and hydrophilic APIs can be entrapped and delivered, for example in liposomes. Phospholipids are nowadays successfully used in numerous approved and marketed depot formulations and are being investigated in ongoing research and development projects.
Formulation options are manifold, ranging from injectable drug crystal suspensions, in which phospholipids are used as dispersing agents, to multivesicular liposomes, in situ formed depots, preformed, aggregated liposomes, and solid depots. These formulations allow optimization towards a zero-order release profile and reduction of burst release effects (Fig. 1). Compared to polymer depots, phospholipid depots exhibit favorable degradation profiles related to the formation of less acidic degradation products. Taken together, this makes phospholipids highly interesting excipients for injectable depot products.
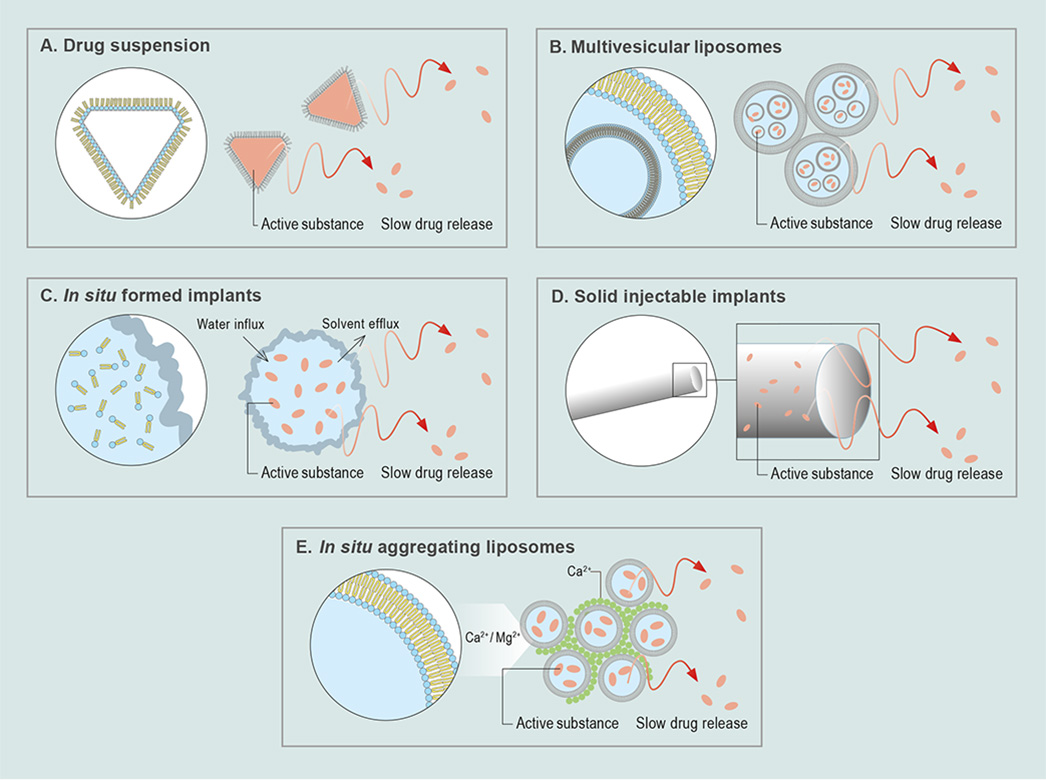
Fig. 1: Overview of categories of phospholipid-based technologies: (A) Drug suspension, (B) multivesicular liposomes, (C) in situ formed depots, (D) solid depots comprising injectable extrudates and (E) in situ aggregating liposomes.
Marketed Depot Injectables with Phospholipids
Phospholipids are proven versatile key excipients in marketed depot injectables based on different technologies such as aqueous drug suspensions, large multivesicular liposomes (DepoFoam®), or in situ formation of a depot matrix at the injection site (FluidCrystal®). Furthermore, mixtures of phospholipids with polymers are used to extend the drug release period (PLEX) and phospholipids are used to coat medical devices such as balloons catheters (NANOLUTE) (Table 1).
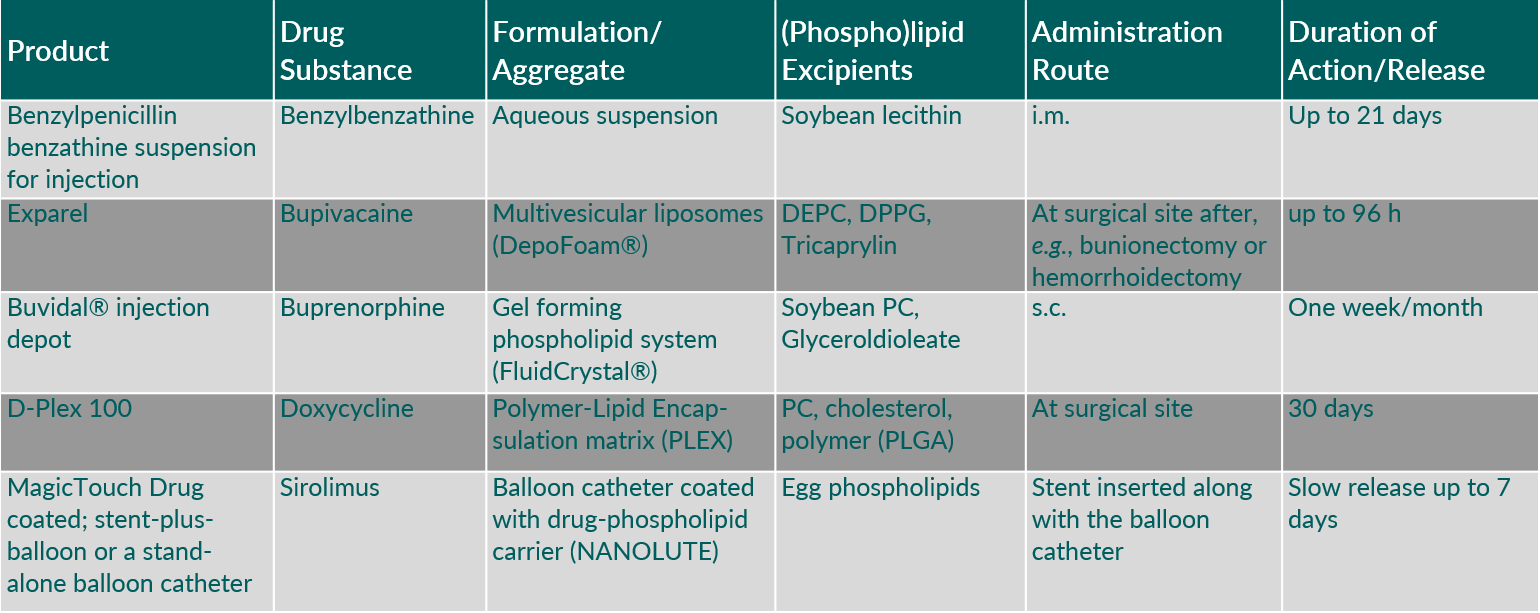
Tab. 1: Characteristics of marketed depot injectables.
Clinical Research Stage Depots with Phospholipids
Recent clinical research efforts with phospholipid-based depot injectables focus on liposomes and in situ formed lipid matrix depot injectables. Polymers may be added to the liposomal depot to extend the drug release period (Table 2).
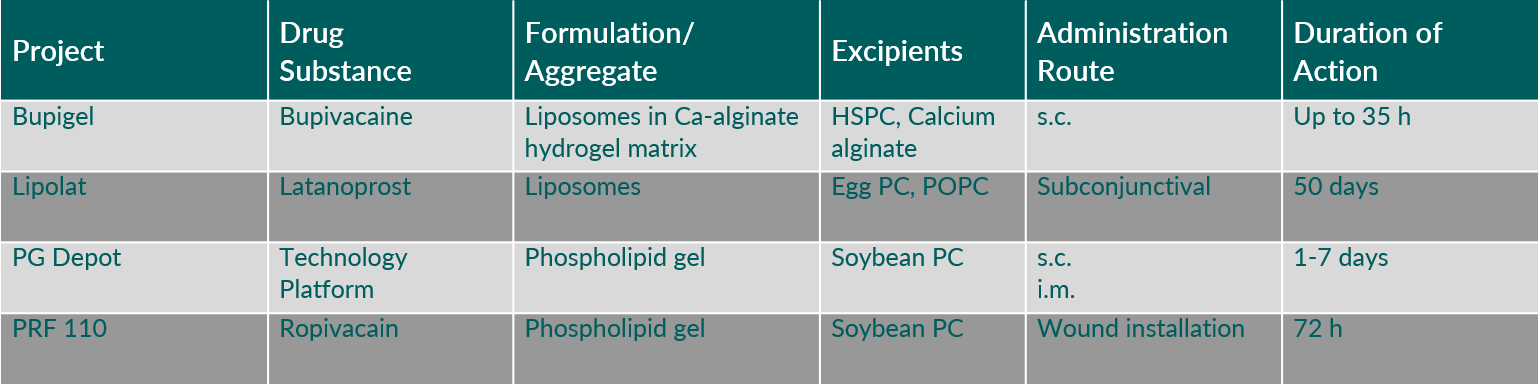
Tab. 2: Examples of phospholipid-based depot systems which are topics of clinical research.
Explorative Depot Technologies with Phospholipids
Recently a depot technology was explored, which used preformed solid injectable implants consisting of phospholipid matrices produced by extrusion (Fig. 1D). Another explorative formulation approach is the use of larger liposomal aggregates with slow-release properties prepared in situ by mixing sterile filtered negatively charged liposomes containing, for example, phosphatidylglycerol (PG) with divalent cations such as Ca2+, prior to administration (Fig. 1E).3) Such slow-release implant matrices allow for drug release periods of several weeks after injection or local administration. Advantages of solid phospholipid implants compared to liquid colloidal systems are ease of manufacture and stability during storage.4)
Concluding Remarks
Phospholipids are unique biocompatible excipients for the development of depot injectables. The broad range of natural and synthetic phospholipids enables the development of depot injectables with a variety of drug release characteristics. The applicability in marketed depot injectables as well as ongoing research projects underscore the suitability of phospholipids for various depot injectable technologies. Research on phospholipid-based depot injectables is rapidly expanding. Besides more sophisticated liposome technology, viscous phospholipid gels and solid phospholipid implants are filling the gap in the field of depot injectables with a focus on intermediate drug release periods of days to several weeks. Besides already used polymer-based depot systems, phospholipid-based depot technologies will open new avenues in extended drug release injectables.
Key advantages of phospholipids in depot formulations:
- Extended drug release covering an intermediate release interval of hours up to several weeks
- Diverse formulation options such as different liposomal formulations, in situ forming depots, and preformed solid as well as semisolid injectables
- Optimization in terms of degradation and drug release profile
- Suitable for lipophilic and hydrophilic drugs, including peptides and proteins
Recent Advances in the Use of Phospholipid Excipients in Local or Injectable Depot Formulations
Pharm. Ind. 8, 1104,
| Google Scholar |
Study on the in situ aggregation of liposomes with negatively charged phospholipids for use as injectable depot formulation
Colloids Surf. B 168, 10-17.
| PubMed |
Nicardipine Loaded Solid Phospholipid Extrudates for the Prevention of Cerebral Vasospasms: In Vitro Characterization
Pharmaceutics 12, 817.
| PubMed |


