Acthera – Unleashing the Power of Mechanosensitive Liposomes
Prof. Dr. Thierry Fumeaux, Dr. Cyrille Maugeais, Prof. Dr. Pierre-Alain Monnard, and Dr. Andreas Zumbuehl, Acthera Therapeutics AG, Rosental Mitte WRO-1059, Mattenstrasse 22, 4058 Basel, Switzerland
Abstract
Liposomes have the potential to revolutionize medicinal treatments by targeting drugs to the diseased tissues or organs. This promise of locally and temporally controlled drug delivery is facing many challenges in human patients. A new drug-targeting approach is developed based on the changes in the blood hemodynamics between healthy and diseased tissues. In this newsletter, we present Acthera Therapeutics Ltd. and our unique next generation of liposomes: Hard-Shelled Liposomes. These liposomes can release their payload upon mechanical stimulation and open the field of mechanosensitive drug delivery.
Acthera Introduces Mechanosensitive Drug Delivery
The human body operates in a complex interplay of biology, chemistry, and physics. Any disease is essentially bringing imbalance into the system. These pathological alterations of body physics may serve as an approach to “sense” the disease and achieve a controlled drug delivery. For instance, a stenosis in a human artery changes the flow dynamics of the blood, leading to the generation of high shear stresses at the artery constriction. These pathological high forces can be utilized for drug delivery by developing mechanosensitive drug delivery systems.1)2)
The principle of mechanosensitive-based drug targeting would be to release a drug payload when a given trigger surpasses a threshold value and to stop releasing its content once the triggering force has dropped below this same threshold value. These alterations of body physics can stem directly from the pathology or, in principle, be artificially created by the local application of energy from outside. The potential of mechanosensitivity with its unique spatio-temporal control over drug delivery is therefore unprecedented.
To produce mechanosensitive liposomes, Acthera Therapeutics Ltd. utilizes an artificial 1,3-diamidophospholipid (Fig. 1) that forms interdigitated gel-state membranes (Fig. 2) at body temperature.3) Faceted Hard-Shelled Liposomes (HSLs) are formed by this lipid, which react to high shear forces by releasing their encapsulated payload molecules. In the absence of unusual high trigger forces, the payload stays encapsulated. This binary behavior allows us to propose the HSL technology as a next generation drug delivery medicinal product with a targeting mechanism based on hitherto unused physics-based mechanisms.
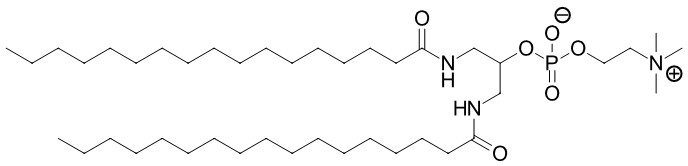
Fig. 1: The artificial 1,3-diamidophospholipid RPCR is at the foundation of the mechanosensitive HSL technology.
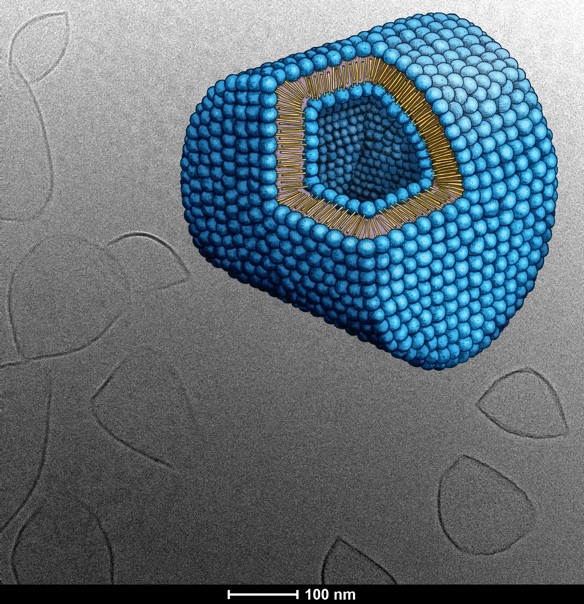
Fig. 2: 1,3-Diamidophospholipids will form interdigitated gel phase liposomes that are non-spherical. The cryoTEM picture shows Hard-Shelled Liposomes and a graphical rendering of the d-form type liposome formed by the 1,3-diamidophospholipids. The unique shape of the d-form liposomes has been derived from cryoTEM tomography images
To fully unleash the power of mechanosensitive, actuated therapeutics, the biotech start-up Acthera Therapeutics Ltd. was launched in Fall 2019. The company unites talents from the field of basic and applied liposome research, preclinical and medical fields, as well as business development. Our vision is to develop mechanosensitive HSLs into novel drug delivery systems that render drugs more efficient to the benefit of the patients by increasing the dose reaching the diseased area while reducing side effects by keeping unwanted interactions between the drugs and the healthy body at the lowest possible level
Acthera’s HSL Technology in vivo
The concept of mechanosensitive HSLs has already been partially demonstrated in our first animal experiments. Here, rat models to study stenosis and thrombus formation in the carotid artery and Eptifibatide as HSL payload were selected. Eptifibatide, an anti-platelet drug (GPIIb/IIIa antagonist) used in clinics, inhibits the thrombus growth in the artery and the obstruction of thereof, hereby maintaining the blood circulation in the affected artery. As Eptifibatide interferes with the blood coagulation cascade, major and minor bleedings are one of its serious side effects. That is, Eptifibatide is a typical example of a drug that would benefit from the mechanosensitive local release.
The formation of a thrombus in the rat carotid artery was chemically induced and concomitantly leading to the increase in shear stress forces at the diseased site. These forces could be harnessed by HSLs containing Eptifibatide. An excellent efficacy was obtained consistent with shear stress-targeted release of Eptifibatide from HSLs (Fig. 3). The efficacious drug dose was substantially lower (at least by more than 66 %) than when Eptifibatide was administered systemically (as a free drug). Additional supporting experiments in vivo confirmed the absence of payload release from HSLs during the injection or within the circulation of healthy animals.
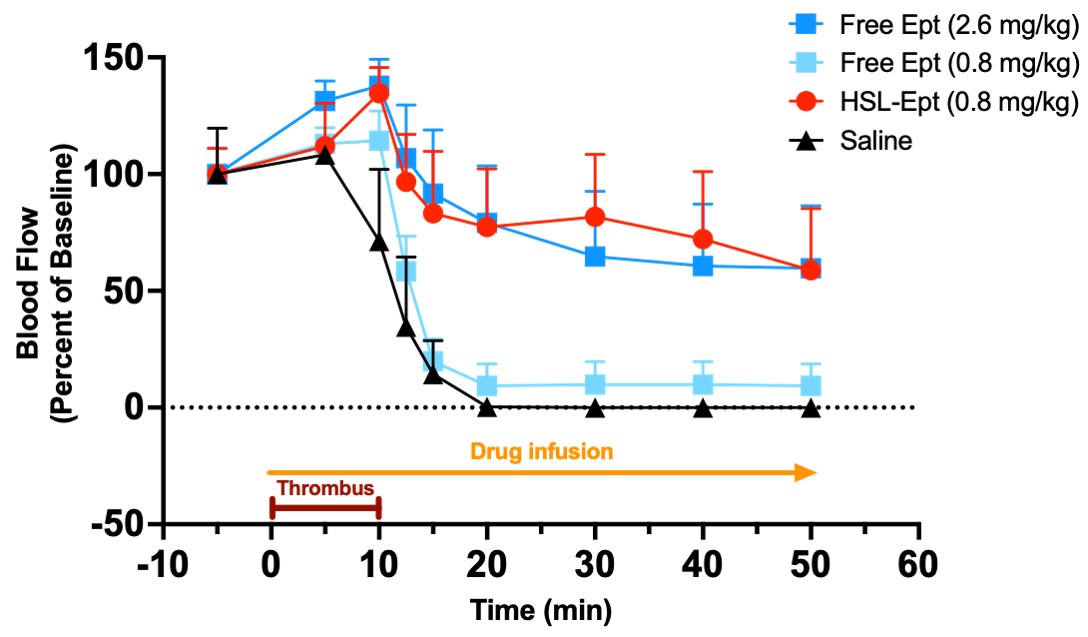
Fig. 3: Targeted release of the GPIIb/IIIa antagonist Eptifibatide triggered by shear stress. The intravenous infusion of free Eptifibatide solution (free Ept) or of an HSL suspensions with Eptifibatide, as payload, (HSL-Ept) was started at the time of the induction of the thrombus formation (brown line) and continued for the whole measurement period (yellow line). The black curve/triangles show the blood flow that was lowered by the formation of a thrombus and completely stopped when the thrombus occluded the artery. The systemic application of free Eptifibatide at sub-therapeutic doses (turquoise squares/curve) did not prevent the occlusion of the artery, as the drop of the blood flow shows. Only a high dose of the free drug (systemic administration, dark blue squares/curve) resulted in the maintenance of the blood flow. Eptifibatide released from HSLs showed the same therapeutic effect, but a substantial reduction of drug payload needed to achieve a therapeutic effect was found and all results point to a successful demonstration of the concept of mechanosensitive drug delivery at the site of the induced thrombus only.
Acthera’s Future Perspectives
The first animal experiment has shown the unique potential of mechanosensitive drug delivery. Acthera will continue spearheading the use of physical triggers for local drug delivery with a pipeline of truly impactful drug payloads. We are looking forward to discussing mechanosensitive drug delivery with you and are strongly supporting the future of the phospholipid and liposome community to the benefit of the patients.
Shear-stress sensitive lenticular vesicles for targeted drug delivery
Nat. Nanotechnol. 7, 536-543.
| PubMed |
Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels
Science 337, 738-742.
| PubMed |
Synthesis and Biophysical Characterization of an Odd-Numbered 1,3-Diamidophospholipid
Langmuir 34, 3215-3220.
| PubMed |


