Registration of Phospholipids and other Lipids as Active Pharmaceutical Ingredients and Excipients in China
In China, the regulatory requirements for registration of pharmaceutical raw materials and active pharmaceutical ingredients (API) have to be considered and adopted for each product. The registration dossier contains information on the quality and manufacture of the raw materials used. Specific requirements have to be fulfilled and the structure and content of the dossier is strictly defined. Phospholipids as pharmaceutical excipients have to be registered together with the drug product producer (bundling review).
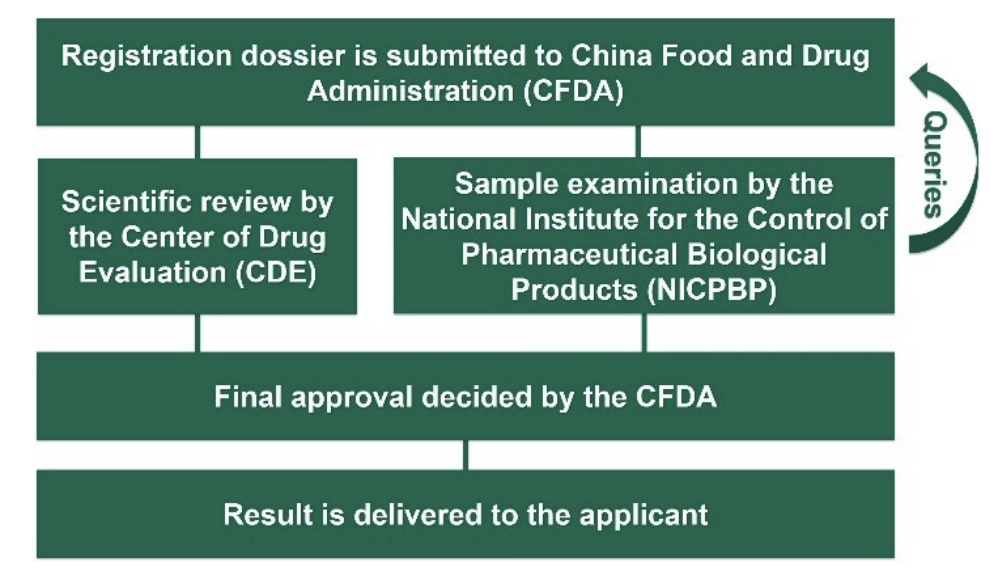
General registration procedure:
Announcement issued by CFDA on matters in relation to bundling review and approval of packaging materials and pharmaceutical excipients (No. 134 in Year 2016)
China as an emerging market is of great interest for the pharmaceutical industry. The regulatory requirements for registration of Active Pharmaceutical Ingredients (API) and excipients are constantly changing. Therefore, the more or less specific requirements in regards to the registration of pharmaceutical raw materials and APIs have to be considered and adopted for each product. Basically, all documents have to be registered at the corresponding competent authorities. The registration dossier should contain information on the quality and manufacture of the raw materials used.
In China, the registration shall be done by the company branch or an entrusted agency on site. It is essential to translate the documents and follow up of the registration proceeding continuously. If an external agency is used, the respective agent is the contact person between registration authorities and the foreign applicant. The registration dossier has to be submitted to the CFDA (China Food and Drug Administration) in Beijing. The documents for API registration will be checked. Then the evaluation of the submitted documents starts through several official bodies, until the final approval is done by the CFDA.
A new regulation for excipient registration in China came into force in 2016 replacing the former Import Drug License (IDL) registration. The most important change in the registration procedure is that the excipient now has to be registered bundled together with the drug product and cannot be registered alone anymore. The structure and content of the registration dossier is strictly defined. These standards have to be followed exactly, representing the major challenge for regulatory affairs departments.
Challenges
- Changing contacts prolonged the registration period
- Clarification and answering of queries from the CDE/NICPBP
- Agreements with costumers for bundled registration
Tips
- Send experts to the test center or CDE and discuss
open issues - Local agency as contact (e.g. manage the communication, track the registration status)

