The unique and different types of phospholipids
There are several types of phospholipids. This article answers the question "What are phospholipids?" and describes the different phospholipid types.
What are phospholipids?
Phospholipids are unique and versatile molecules. They occur naturally and represent the main components of cellular membranes. Arranged in a lipid bilayer, they play a crucial role in the structure and function of biological membranes. In addition, they participate in many cellular processes and are used as excipients in pharmaceutical formulations.
Phospholipids are amphiphilic molecules, meaning they have both water-loving (hydrophilic) and fat-loving (lipophilic) parts. Structurally, they are esters of glycerol. The sn-1 and sn-2 positions of the glycerol backbone are esterified with fatty acids of varying chain length and saturation (shown in symbolic drawings as yellow sticks). The sn-3 position, in contrast, is esterified with phosphoric acid, which itself is esterified with a short-chain alcohol. This alcohol determines the hydrophilic headgroup of the phospholipid (illustrated as a blue ball, see Figure 1).1)
For example, the systematic name of phosphatidic acid (PA) is 1,2-diacyl-sn-glycero-3-phosphate. The abbreviation “sn” refers to stereospecific numbering. It indicates that the carbon atom that appears on top in the Fischer projection showing a vertical carbon chain with the hydroxyl group at carbon-2 to the left is designated as C-1.2)
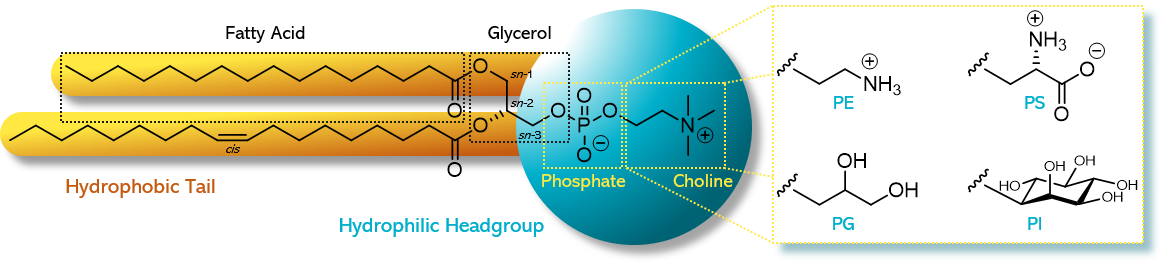
Figure 1. Chemical structure of a phospholipid as exampled by 1-palmitoyl-2-oleoyl-sn-glycero-3phosphocholine (POPC) and the different alternative headgroups varying in the type of alcohol: phosphatidylethanolamine (PE) with ethanolamine, phosphatidylglycerol (PG) with glycerol, phosphatidylserine (PS) with serine, and phosphatidylinositol (PI) with inositol. Figure adapted from Drescher S, van Hoogevest P, 2020. The Phospholipid Research Center: Current Research in Phospholipids and their Use in Drug Delivery, Pharmaceutics 12, 1235.1)
Classification of phospholipids
The structure of alcohol attached defines the different types of phospholipids. Examples include phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), and phosphatidylserine (PS).3) In mammalian plasma membranes, the four predominant phospholipids are PC, PE, PS, and sphingomyelin.
The arrangement of substituents on the sn-1, sn-2, and sn-3 positions introduces chirality. Depending on the polar headgroup and the surrounding pH, PE and PC are zwitterionic and carry a neutral charge at pH 7. In contrast, PG, PI, and PS are negatively charged under the same conditions (see Figure 2).
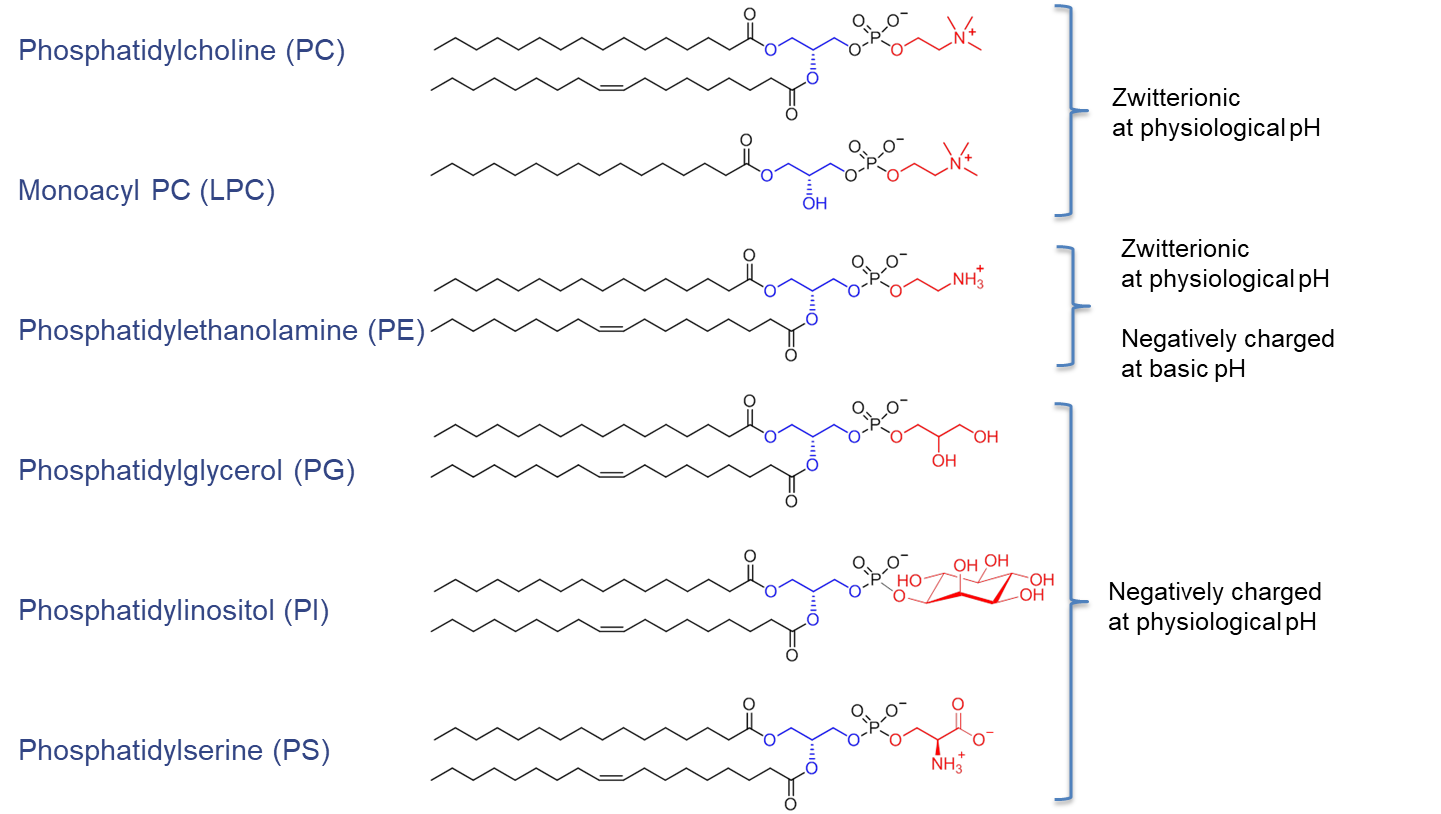
Figure 2. Chemical structure of phospholipids (blue, glycerol; red, alcohol) and their net charge at physiological pH value. Figure adapted from van Hoogevest P, Tiemessen H, Metselaar JM, Drescher S, Fahr A, 2021. The Use of Phospholipids to make Pharmaceutical Form Line Extensions, Eur. J. Lipid Sci. Technol., 2000297.4)
Nomenclature of phospholipids
Phospholipids with two esterified fatty acids are called diacyl-phospholipids. If only one fatty acid is present, they are called monoacylphospholipids or lyso-phospholipids.
In scientific literature, synthetic phospholipids are often abbreviated according to this scheme: position of fatty acid – type of fatty acid – phosphatidyl – alcohol. For example, “POPC” stands for 1-palmitoyl-2-oleoylphosphatidylcholine (IUPAC: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine). Another example is “DOPE” for 1,2-dioleoylphosphatidylethanolamine (IUPAC: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine).
Because the number, type, and position of fatty acids and the headgroup can vary, nature provides an extremely large diversity of phospholipids. Furthermore, the fatty acid composition depends on the biological source, whether plant or animal.
For additional details, see our phospholipid publications.
Phospholipid, Lecithin, Phosphatidylcholine - what is what?
It is important to distinguish the terms "phosphatidylcholine" and "lecithin". According to the United States Pharmacopeia (USP), lecithin is defined as “a complex mixture of acetone-insoluble phosphatides (i.e., phospholipids), consisting mainly of PC, PE, PI, and PA, in combination with various amounts of other substances such as triglycerides, fatty acids, and carbohydrates, as separated from the crude vegetable oil source. It contains not less than 50% acetone-insoluble matter.”
However, especially in American literature, lecithin is sometimes incorrectly used as a synonym for pure phosphatidylcholine. If a product is described only as containing “lecithin,” it remains unclear which specific lipid is present.
Therefore, the following terminology is recommended:
-
Use "lecithin" only when a product contains less than 80% phospholipids by weight.
-
Use "phospholipid" for products containing 80–100% phospholipids (e.g., PC, PE).
-
Name the specific phospholipid if its content exceeds 90% of the product weight.1)
You want to know more?
Please use the following links:
What types of phospholipids are there?
What is the occurrence and benefit of phospholipids?
What phospholipid aggregates are formed?
Which pharmaceutical formulations can be made?
The Phospholipid Research Center: Current Research in Phospholipids and their Use in Drug Delivery
Pharmaceutics 12, 1235
| PubMed |
Nomenclature of phosphorus-containing compounds of biochemical importance (Recommendations1976)
Proc. Natl. Acad. Sci. USA 74, 2222
| Pubmed |
The Use of Phospholipids to make Pharmaceutical Form Line Extensions
Eur. J. Lipid Sci. Technol., 2000297
| Wiley |

