A hydrogen peroxide-sensing liposomal hydrogel for molecular diagnostics in diabetic wounds
Prof. Dr. Simon Matoori1) – Université de Montréal, Canada and Prof. Dr. Aristidis Veves2) – Beth Israel Deaconess Medical Center, a Harvard Medical School Teaching Hospital, USA
1.
2.
People involved
Gabriela Manrique (PhD fellow funded by the PRC) - Université de Montréal
Katia Cherifi (MSc student) - Université de Montréal
Abstract
Diabetic foot ulcers (DFUs) are a serious complication of diabetes. DFUs affect 15-25% of patients with diabetes in their lifetime and are a major cause of amputations in industrialized countries.1) The standard of care in DFU diagnostics is a macroscopic evaluation (wound size, depth, signs of infection).2) In the future, molecular diagnostics promise to enable molecular disease staging, evidence-based treatment selection, and assessment of treatment success. Recent advances in the understanding of diabetic wound healing indicate the key role of hydrogen peroxide (H2O2) in the pathophysiology of diabetic wound healing.3)4)5) H2O2 is therefore a particularly promising molecular diagnostic target in DFU.
The overall goal of this research proposal is to develop fluorescent H2O2-sensing diagnostic wound dressing, and to test it in diabetic mouse wounds using a portable fluorometer. The dressing is composed of H2O2-sensing liposomal reaction compartments dispersed in an alginate hydrogel. After administration on the wound, H2O2-rich wound fluid diffuses into the hydrogel. H2O2 enters the liposomal lumen where it is used by horseradish peroxidase (HRP) to oxidize the near-infrared fluorescent dye sulfo-cyanine 7 (S7), which results in an H2O2 concentration-dependent loss of fluorescence. The fluorescence decrease is detected by a portable fluorometer. To prolong shelf-life, a cryo- and lyoprotectant-containing formulation and a lyophilization process will be developed to dry the liposomal hydrogel with minimal cargo release. This highly specific in situ wound H2O2 assay is tested in diabetic mouse wounds in vivo, as we ultimately aim to develop a rapid and easy-to-use wound H2O2 assay to enable molecular diagnostics in DFU at the point-of-care.
Developing a liposomal hydrogel formulation with selective membrane permeability (high permeability for the analyte H2O2 and low permeability for the assay components HRP and S7) and high stability in the wound environment (varying pH values, proteins) coupled with a lyophilization procedure to prepare a dried formulation will be of high interest to the phospholipid community.
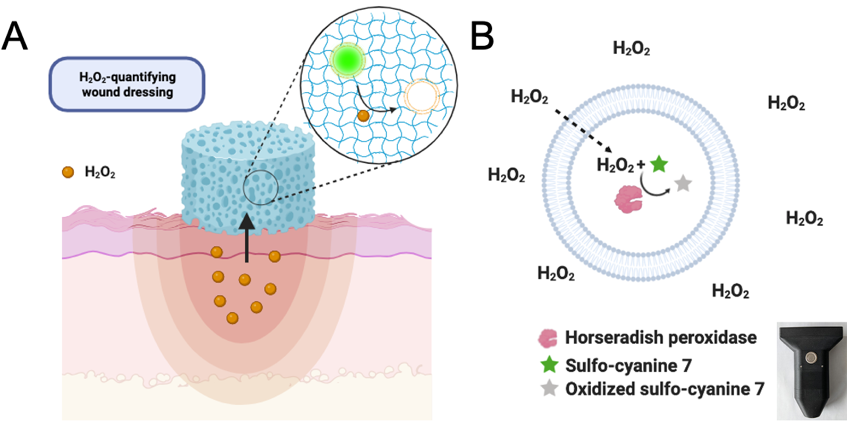
Figure 1. Schematic depiction of H2O2-sensing liposomal hydrogel. The diagnostic wound dressing consists of fluorescent liposomes immobilized in a hydrogel scaffold (A). After administration of the hydrogel on the wound, wound H2O2 diffuses into the hydrogel and the liposomes. H2O2 readily diffuses across the liposomal membrane due to its small size and neutral charge. Once inside the lumen, H2O2 is used by the enzyme horseradish peroxidase (HRP) to oxidize the near-infrared fluorescent dye sulfo-cyanine 7 (S7), leading to a loss of fluorescence (B). The fluorescence change is detected in situ by a portable fluorometer (insert).
Benefit for the community
Phospholipid research community
The liposomal hydrogel formulation development will generate new knowledge about selectively permeable membranes, and the stability of liposomes in hydrogels and liposomal hydrogels in biological fluids (wound fluid). Developing a dried liposomal hydrogel formulation, we will provide cryo- and lyoprotectant-containing formulations and freeze-drying procedures with high cargo retention to the community. This information is of high interest regarding the scarcity of literature in lyophilized liposomal hydrogels. The generated knowledge will serve the emerging field of liposomal hydrogels in diagnostics, drug delivery, and biomedical and tissue engineering.
DFU patients
There is an unmet clinical care gap for molecular diagnostics in DFU.2) The H2O2-sensing wound dressing could enable molecular disease staging, the identification of patients with high wound H2O2 who will benefit most from H2O2-lowering treatment, and the assessment of treatment response on a molecular level, and thus enable personalized medicine in DFU.
Visit the supervisors lab
Prof. Dr. Simon Matoori (Université de Montréal)
Prof. Dr. Aristidis Veves (Beth Israel Deaconess Medical Center, a Harvard Medical School Teaching Hospital)
Early controlled release of peroxisome proliferator-activated receptor β/δ agonist GW501516 improves diabetic wound healing through redox modulation of wound microenvironment
J. Controlled Release 197, 138-147
| PubMed |
Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters
J Diabetes Res 2014, 562625
| PubMed |
The redox enzyme p66Shc contributes to diabetes and ischemia-induced delay in cutaneous wound healing
Diabetes 59, 2306-2314
| PubMed |

