(Mixed) Micelles
Micelles are formed by one type of amphiphilic molecule with a cone shape like lyso phospholipids, whereas mixed micelles contain two or more amphiphilic molecules. The amphiphilic molecules self-assemble into colloids with a hydrophilic shell formed at the interface with water by the polar heads and a hydrophobic core formed by the lipophilic parts.
Physiologically, mixed micelles arise in the small intestine after the ingestion of fats and secretion of pancreatic and biliary juices.1) Lipophilic nutrients are solubilized in their hydrophobic core and can be absorbed.
Pharmaceutical applications
Commercially available mixed micellar products
B. Moore was the first to mention mixed micelles in the literature in 1909.2) In 1916, H. Wieland described the use of mixed micelles to solubilize poorly water soluble drugs.3) In the 1960s, mixed micelles containing a phospholipid with polyunsaturated fatty acids were introduced. In the 1970s, a mixed micellar formulation for the solubilization of diazepam was reported.
Since then, these formulations were used in several injectable products to solubilize poorly water-soluble drugs or vitamins. In addition, there are injectable mixed micellar products on the market with phospholipids comprising essential fatty acids as active. They are used, e.g., for regulation of plasma lipid levels and liver protection.
Mixed micellar formulations can be prepared by dissolving the phospholipid component in an aqueous solution of the bile salt, followed by dissolving the drug substance. Alternatively, a blend of phospholipid and bile salt, with or without drug substance can be manufactured by drying from organic solvent or aqueous solutions, followed by hydration of the dry blend. The aqueous mixed micellar formulation is then sterilized by means of sterile filtration and when needed lyophilized. Typically, soybean phosphatidylcholine and cholate salts are used to prepare pharmaceutically applied mixed micelles. Minimum weight ratios of bile salt to phospholipid of 0.7-0.8 are needed to obtain clear solutions. 4)
Mixed micelles are predominantly administered intravenously but are also used as oral dosage form, even for pediatric applications. They are easy to produce, stable upon storage and well tolerated after administration.5) Table 1 provides examples of mixed micellar products that are currently on the market.
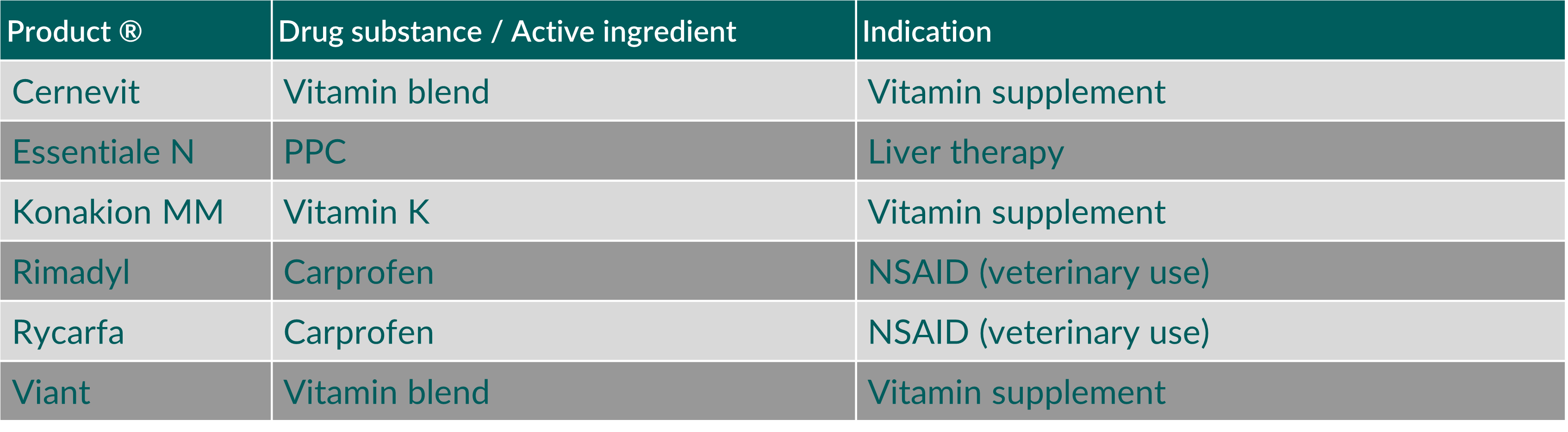
Table 1. Marketed mixed micellar products containing soybean phosphatidylcholine as phospholipid component. (Abbreviations: NSAID, nonsteroidal anti-inflammatory drug; PPC, polyenyl-phosphatidylcholines)
Research on (mixed) micelles
After intravenous administration, mixed micelles may transform, e.g., because of dilution and presence of plasma proteins. The unclear fate of mixed micelles and consequently, also their cargo hampers a wider use as drug delivery system.6)
In the gastrointestinal tract, mixed micelles form as a result of digestion after oral ingestion of lipid-based formulations such as liposomes. Hence, compound transfer between administered formulations and colloids arising after their administration is an important research subject for this administration route.
Various PRC-funded projects investigate mixed micelles, e.g., their fate after administration or their use in oral pediatric formulations.
You want to know more about phospholipid-based formulations?
Please use the following links:
What applications are there for phospholipid-based emulsions?
How do phospholipids influence the characteristics of liposomes?
What role do phospholipids play in lipid nanoparticles (LNPs)?
Biological fate of ingested lipid-based nanoparticles: Current understanding of future directions
Nanoscale 11
| PubMed |
An evaluation of the safety of mixed micelles in healthy subjects.
J. Parenter. Enteral Nutr. 20(2):110-112.
| PubMed |
Investigations on Mixed Micelle and Liposome Preparation for Parenteral Use Based on Soya Phosphatidylcholine
Eur J Pharm Biopharm 40(3): 147-156.
| Google Scholar |
Reflection paper on the pharmaceutical development of intravenous medicinal products containing active substances solubilised in micellar systems
| EMA |

