Lipid nanoparticles (LNPs)
Lipid nanoparticles (LNPs) gained prominence as the delivery system for messenger RNA (mRNA) in vaccine formulations for COVID-19. Before that, LNPs were introduced for intravenous administration of small interfering RNA (siRNA) in the treatment of rare diseases.
Learn more about this emerging delivery system here.
Excipients used for lipid nanoparticles
Typically, LNPs comprise around 50 % of the ionizable lipid, 1.5 % of a PEGylated lipid, 38.5 % of cholesterol, and 10 % of the synthetic phospholipid 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC).1) This lipid matrix provides a protective shell that shields the nucleic acid from nucleases and serves as a shuttle so that the nucleic acid can pass the cell membrane.
- The ionizable lipids DLin-MC3-DMA, ALC-0315, and SM-102 are used in commercialized LNP products. They contain a tertiary amine with apparent pKa values around 6.5 and a truncated cone shape, that are essential characteristics for complexation of nucleic acids and endosomal escape. The later released ionizable lipids ALC-0315 and SM-102 provide in addition a cleavable ester structure, resulting in rapid clearance of these lipids from the injection site.
- The PEGylated lipid contributes to the submicron size and confers bio-inert properties.
- The role of phospholipids and cholesterol are presumed to contribute to LNP structure and stability. However, the versatile physicochemical properties of different phospholipids inspire ongoing research on their role and how they can modulate the properties of LNPs.
Pharmaceutical applications of lipid nanoparticles
LNPs are a relatively new delivery system compared to liposomes, emulsions and (mixed) micelles. Onpattro, a siRNA-containing LNP, was approved in 2019, quickly followed by the mRNA vaccines.2)
Table 1 summarizes parenteral products containing synthetic phospholipids used for vaccines and RNA delivery.
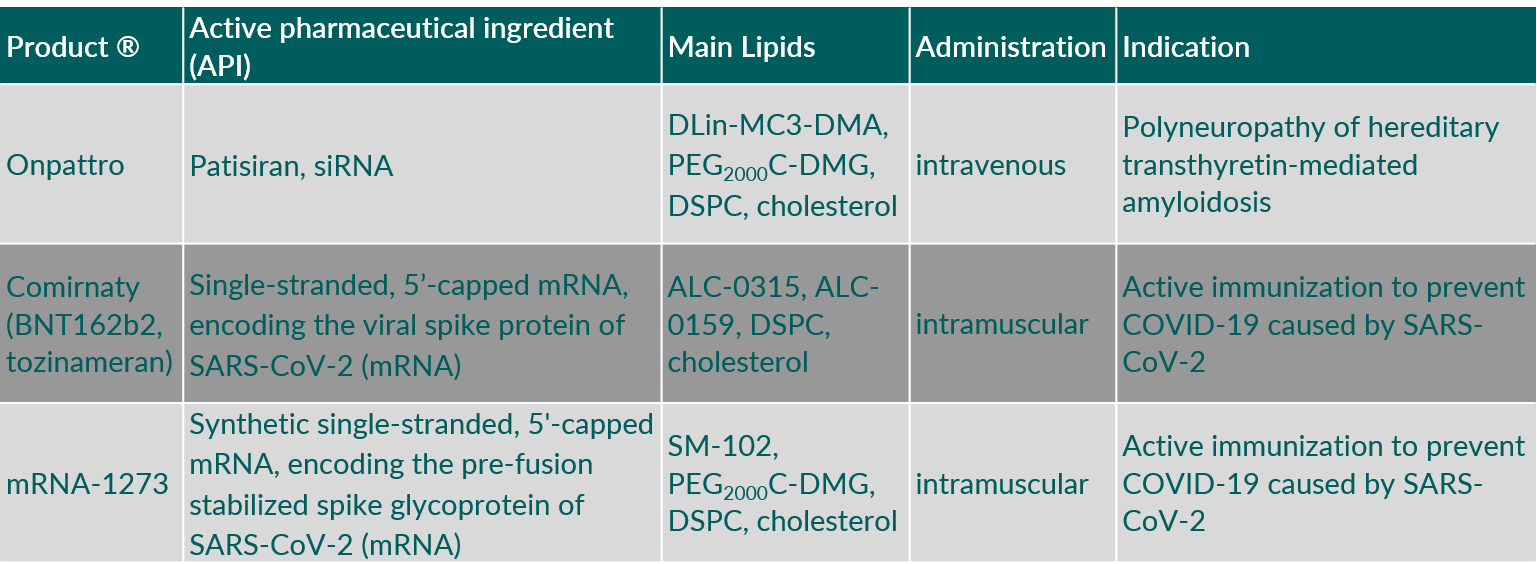
Table 1. Parenteral products containing synthetic phospholipids used for vaccines and RNA delivery. (Abbreviations: ALC-0159, 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide; ALC-0315, ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate); DLin-MC3-DMA, (6Z,9Z,28Z,31Z)-heptatriaconta-6,9,28,31-tetraen-19-yl-4-(dimethylamino)butanoate; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; PEG2000C-DMG, 3’-({[1,2-di(myristyloxy)propanoxy]carbonylamino}propyl)-ω-methoxy, polyoxyethylene.
Phospholipid-based research on LNPs
In ongoing research projects, the phospholipid DSPC is substituted or complemented with further phospholipids to tune the properties and performance of LNPs. Phospholipids with a geometry of a truncated cone like phosphatidylethanolamines or ones with a lower phase transition temperature are applied to increase endosomal escape properties.3)4)
Moreover, phospholipids with different charges are applied to alter the biodistribution of LNPs.5) These strategies highlight the versatility of phospholipids and their potential to further enhance the efficacy and broaden the application of these emerging carriers.
You want to know more about phospholipid-based formulations?
Please use the following links:
Which applications exist for phospholipid-based emulsions?
How do phospholipids influence the characteristics of liposomes?
How are mixed micelles formed and applied?
The role of lipid components in lipid nanoparticles for vaccines and gene therapy
Adv. Drug Delivery Rev. 188
| PubMed |
Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs
Drug Metab. Pharmacokinet.
| PubMed |
Optimization of phospholipid chemistry for improved lipid nanoparticle (LNP) delivery of messenger RNA (mRNA)
Biomater. Sci. 10(2)
| PubMed |
Acidification-Induced Structure Evolution of Lipid Nanoparticles Correlates with Their In Vitro Gene Transfections
ACS Nano 17(2)
| PubMed |
Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing
Nat. Nanotechnol. 15(4)
| PubMed |

