Lipospec – Bringing Lipid Order into Focus for RNA Drug Delivery
Katharina Beck and Dr. Nicolas Färber, Lipospec, Werner-von-Siemens-Str. 6, 86159 Augsburg, Germany.
Bringing Lipid Order into Focus for RNA Drug Delivery
In recent years, RNA-based therapeutics have advanced rapidly, with lipid nanoparticles (LNPs) playing a central role in enabling the safe and efficient delivery of these fragile molecules. These lipid-based carriers typically consist of four main components: an ionizable cationic lipid, cholesterol, a phospholipid (e.g., DSPC), and a PEG-lipid.1) Despite their growing popularity, LNP formulations are not yet fully understood. While much attention has been paid to aspects like RNA encapsulation efficiency and release, the structural behavior of the lipids themselves is often neglected. However, a deeper understanding of lipid organization at the molecular level could hold the key to improving the stability, performance and scalability of LNP formulations.
A versatile approach to studying lipid order is the use of polarity-sensitive fluorescent probes such as Laurdan.2) This well-established dye integrates into lipid membranes and reports on the local hydration and packing density of lipids by shifting its emission spectrum. The calculation of the generalized polarization (GP) from this spectral response provides a quantitative measure of lipid order. Laurdan has been widely used in membrane biophysics and fluorescence microscopy for decades.3) Its application to LNP formulations now holds great potential to provide meaningful insights into the lipid structure and dynamics of these modern drug delivery systems.
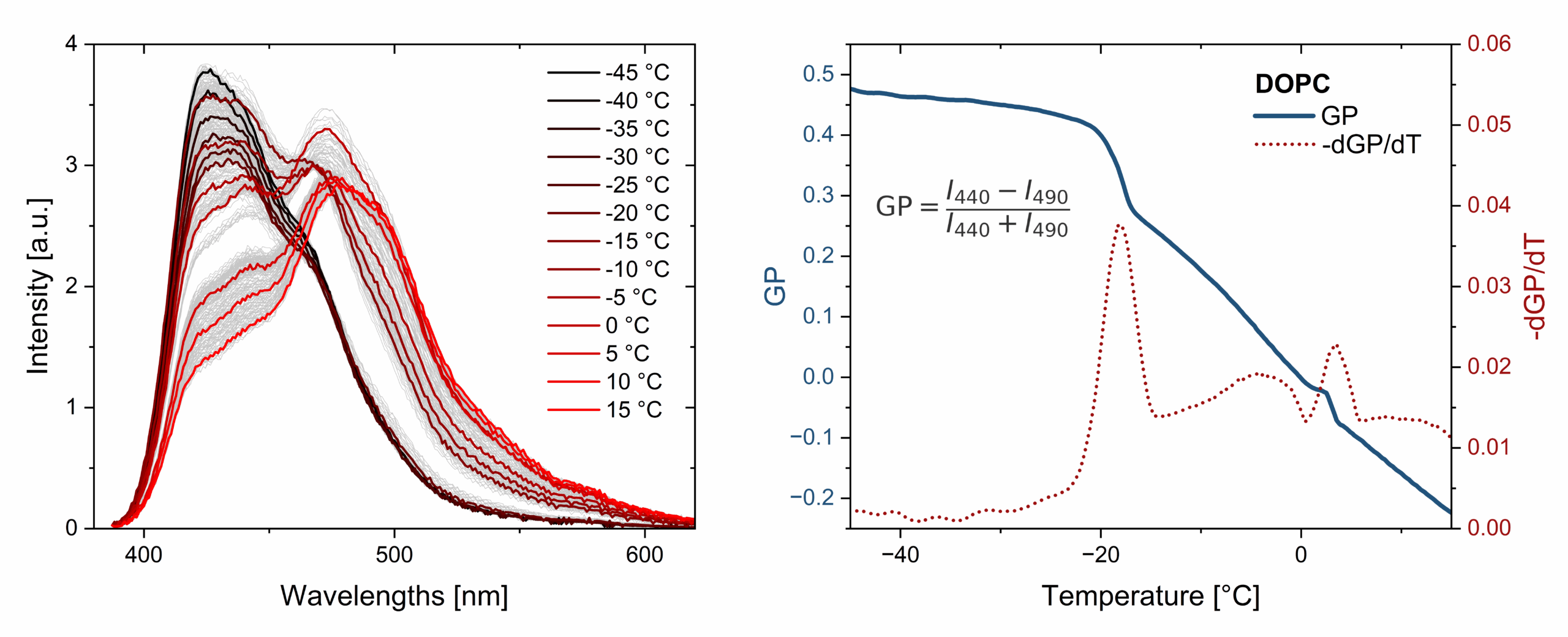
Figure 1: Temperature-dependent fluorescence analysis of multilamellar DOPC vesicles stained with 0.5 mol% Laurdan. Using the Lipid State Observer (LISO), the MLV suspension was analyzed from –40 °C to 10 °C with a heating rate of 2 K/min. Left: Representative fluorescence emission spectra at selected temperatures. Right: Calculated generalized polarization (GP) as a function of temperature (blue curve, left axis), and its negative first derivative (red dots, right axis), indicating a phase transition at –18.1 °C as the point of maximal GP change.
Laurdan Fluorescence Spectroscopy
Applying Laurdan fluorescence spectroscopy to LNP formulations enables the detailed characterization of lipid phase behavior under various physicochemical conditions, including changes in temperature, pH, and ionic strength.4)5)6)7) These factors are known to influence lipid packing and hydration, parameters that are critical for the stability and performance of LNP-based therapeutics.
By measuring GP as a function of temperature, both discrete phase transitions and continuous, temperature-dependent structural rearrangements can be resolved. Furthermore, the method allows for distinguishing between reversible and irreversible temperature-dependent changes in lipid order. Notably, Laurdan fluorescence spectroscopy is suitable for frozen samples,2) permitting the in situ analysis of LNPs under actual storage conditions. In addition, it enables monitoring of structural dynamics during critical temperature transitions, such as freezing after formulation, thawing prior to application, and the subsequent warming to physiological temperature.
Importantly, recent work has demonstrated that alterations in lipid order detected by the Laurdan assay correlate with in vivo transfection efficiency of LNPs,7) highlighting the relevance of Laurdan fluorescence spectroscopy not only for biophysical characterization but also as a predictive tool in formulation optimization during LNP development.
The Lipid State Observer (LISO)
The Lipid State Observer (LISO) is a fluorescence spectrometer specifically designed to assess temperature-dependent lipid order using solvatochromic dyes such as Laurdan.2)8)9)10)11)
Lipid samples (including LNP formulations, liposomes, model membranes, or even living cells) are stained with a fluorescent probe and measured directly in small PCR tubes containing 250 µL of sample. The sample is excited at 360 nm with adaptive integration time, and fluorescence emission spectra are recorded automatically with background correction. The device supports user-defined temperature profiles and repeated runs between -40°C and +90 °C, thereby enabling detailed analysis of temperature-dependent changes in lipid organization, including phase transitions, across a broad temperature range. This includes subzero temperatures, without the need for additional cooling systems such as liquid nitrogen. Dedicated analysis software facilitates the evaluation of raw spectra and calculates GP as function of temperature or time, ensuring that reliable and meaningful data can be obtained even without prior expertise in spectroscopy.

Figure 2: Lipid State Observer (LISO), a fluorescence spectrometer for temperature-controlled measurements to characterize lipid order and phase behavior in systems ranging from lipid-based drug formulations such as LNPs and liposomes to model and living cell membranes, using solvatochromic probes such as Laurdan.
Concluding Remarks
Lipospec was founded as a research-driven spin-off from the University of Augsburg with the aim to make advanced lipid structure analysis broadly accessible for lipid research and especially LNP development and formulation. We are committed to providing tools that combine ease of use with reliable, reproducible results, designed to seamlessly integrate into existing laboratory workflows. With LISO, we proudly introduce our first commercially available instrument specifically designed to measure temperature-dependent lipid order using fluorescence spectroscopy. At Lipospec, we believe that a deeper understanding of lipid behavior is essential to sustainably improve RNA-based drug formulations and accelerate their translation from research to clinical application.
The role of lipid components in lipid nanoparticles for vaccines and gene therapy
Adv. Drug Deliv. Rev. 188, 114416
| PubMed |
Broad lipid phase transitions in mammalian cell membranes measured by Laurdan fluorescence spectroscopy
Biochim Biophys Acta Biomembr 1864, 183794
| PubMed |
Acidic pH-induced changes in lipid nanoparticle membrane packing
Biochim Biophys Acta Biomembr 1863, 183627
| PubMed |
Leveraging Biological Buffers for Efficient Messenger RNA Delivery via Lipid Nanoparticles
Mol. Pharmaceutics 19, 4275–4285
| PubMed |
Membrane fluidity properties of lipid-coated polylactic acid nanoparticles
Nanoscale 16, 8533–8545
| PubMed |
PEGylated lipid screening, composition optimization, and structure-activity relationship determination for lipid nanoparticle-mediated mRNA delivery
Nanoscale 17, 11329–11344
| PubMed |
Microfluidic Transfection System and Temperature Strongly Influence the Efficiency of Transient Transfection
ACS Omega 9, 21637–21646
| PubMed |
Strong Membrane Permeabilization Activity Can Reduce Selectivity of Cyclic Antimicrobial Peptides
J. Phys. Chem. B 129, 2446–2460
| PubMed |
Order disorder phase transitions in the plasma membrane of HeLa cells measured by fluorescent analysis of solvatochromic probes
bioRxiv, 2025.2001.2029.635609
| bioRxiv |