Applications of supercritical carbon dioxide to the production and sterilization of liposomes
Noémie Penoya, Kouka Luc Delmac, Nirmayi Homkar, Abdoul Karim Sakiraf, Sabrina Egrekd, Rosalie Sachelid, Pierre-Yves Sacrée, Bruno Grignardb, Marie-Pierre Hayetted, Touridomon Issa Soméf, Rasmané Semdéc, Brigitte Evrarda, Géraldine Piela
a Laboratory of Pharmaceutical Technology and Biopharmacy, Development of Nanomedicine, Center for Interdisciplinary Research on Medicines (CIRM), University of Liege, Avenue Hippocrate 15, 4000 Liege, Belgium
b FRITCO2T (Federation of Researchers in Innovative Technologies for CO2 Transformation), University of Liege, Sart-Tilman B6a, Liege 4000, Belgium
c Laboratory of Drug Development, Doctoral School of Sciences and Health, University Joseph KI-ZERBO, 03 BP 7021 Ouagadougou 03, Burkina Faso
d Laboratory of Clinical Microbiology, Center for Interdisciplinary Research on Medicines (CIRM), University of Liège, Avenue Hippocrate 15, 4000 Liege, Belgium
e Research Support Unit in Chemometrics, Center for Interdisciplinary Research on Medicines (CIRM), University of Liege, Avenue Hippocrate 15, 4000 Liege, Belgium
f Laboratoire de Toxicologie, Environnement et Santé (LATES), Ecole Doctorale des Sciences de La Santé (ED2S), Université Joseph KI-ZERBO, 03 BP 7021 03 Ouagadougou, Burkina Faso
corresponding author: Noémie Penoy (npenoy@uliege.be)
The Method
Use of supercritical carbon dioxide to develop sterile liposomes co-encapsulating hydrophilic and hydrophobic drugs with compatible physicochemical characteristics for drug delivery.1)2)
A Particle from Gas Saturated Solution (PGSS) (Figure 1) process was developed using a Quality by Design (QbD) strategy. This strategy allowed to determine two production conditions (Table 1) involving different parameters to produce liposomes with compatible physicochemical characteristics for drug delivery (size < 200 nm and PdI < 0.35) in a one-step process and without any use of organic solvents. This production method has been successfully transferred to the production of liposomes of different lipid compositions (Table 2) and to the encapsulation and co-encapsulation of hydrophilic and hydrophobic drugs at encapsulation efficiency reaching to 94% and 40% respectively.
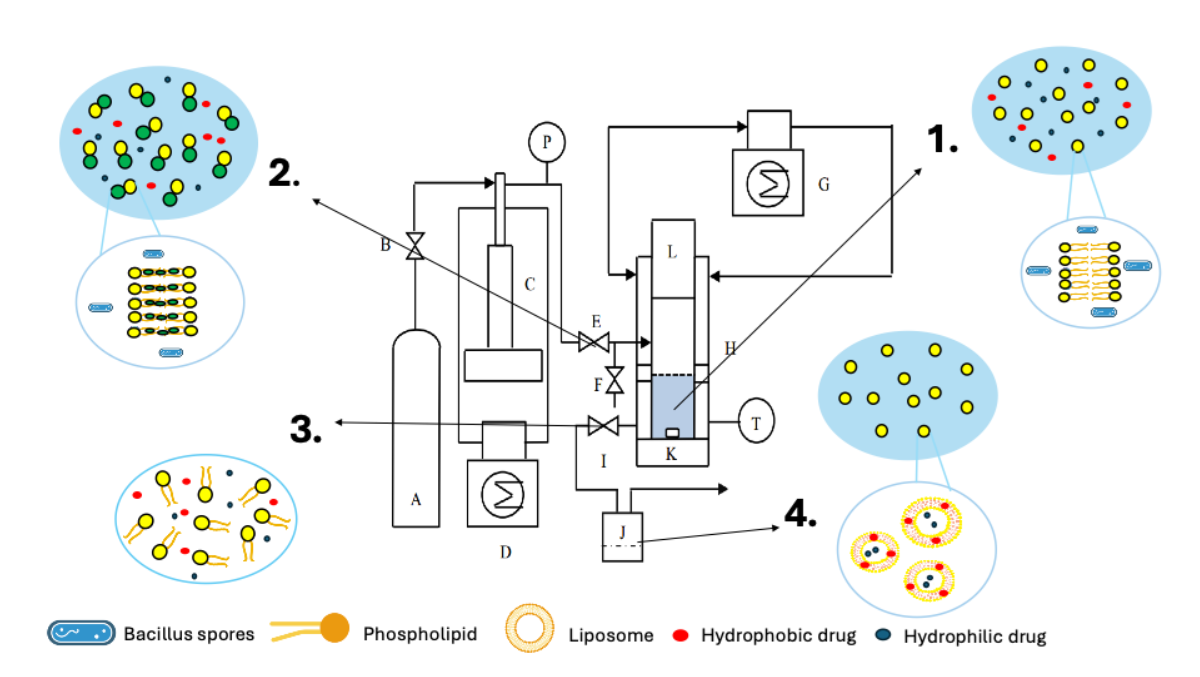
Figure 1: PGSS process. 1) Insertion of a lipid dispersion containing hydrophilic and hydrophobic molecules contaminated with bacillus spores into the PGSS reactor, 2) pressurization of the reactor with supercritical carbon dioxide, 3) depressurization of the reactor, 4) sterile liposomes co-encapsulating hydrophilic and hydrophobic molecules recovery.
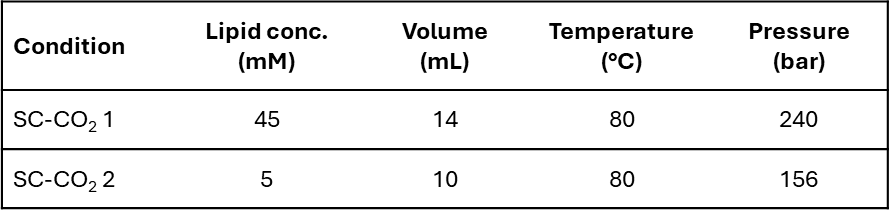
Table 1: Production conditions.
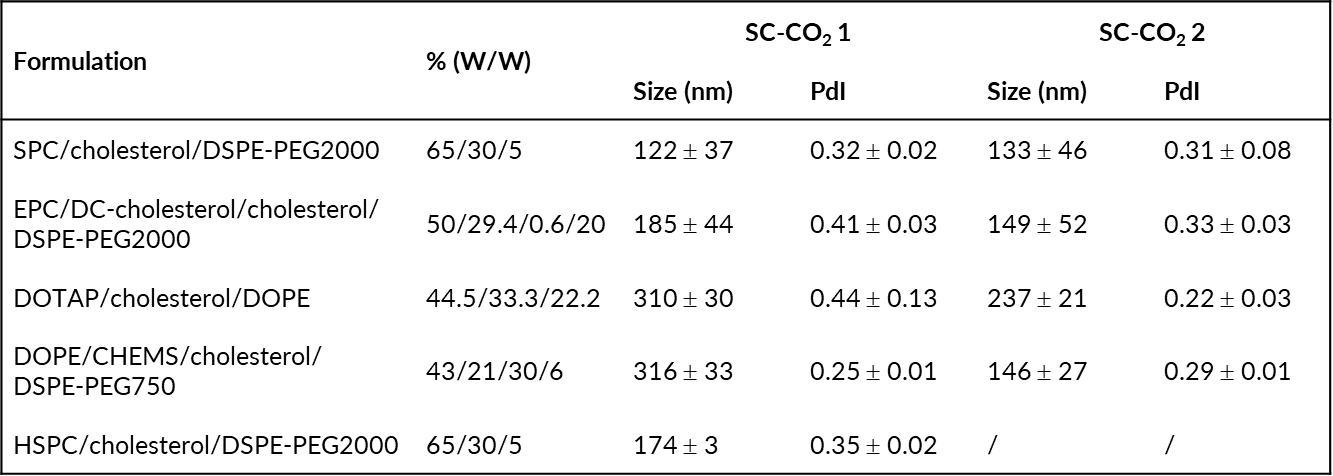
Table 2: Size (nm) and PdI of different liposomal formulations produced by PGSS process under SC-CO2 1 and SC-CO2 2 condition.
Supercritical carbon dioxide has also a great microbicidal potential and represents an interesting alternative for the sterilization of sensitive products such as liposome. By applying another QbD strategy, it was found that the condition SC-CO2 1, involving a temperature of 80°C and a pressure of 240 bar for 30 minutes, allowed to decrease the sterility assurance level (SAL) of contaminated liposomes at a SAL < 6 log which demonstrate that the sterilization process is efficient for pharmaceutical product and to obtain sterile liposomes with compatible physicochemical characteristics for drug delivery in a one-step process, facilitating transfer to industrial scale.
Key Achievements
- Development of a completely one-step process3) without the use of organic solvents or any additives to obtain sterile liposomes with compatible physicochemical characteristics for drug delivery (size < 200 nm and PdI < 0.35 with the required SAL).
- Production of liposomes of different lipid composition adapted for different applications.
- Production of both low (5 mM) and high (45 mM) concentrated batches which is very attractive from an industrial point of view.
- Encapsulation and co-encapsulation of hydrophobic and hydrophilic molecules with high encapsulation efficiencies suitable for industrial perspectives.
Key Competitive Advantages
- Reduction of the number of production steps compared to other conventional production methods or to the industrial method currently used, reducing the production cost and increasing its transferability to a larger scale in GMP conditions.
- Solvent free method compared to other innovative method for liposomes production such as microfluidics which use a large quantity of ethanol.
- The PGSS process allow to obtain the same physicochemical characteristics in a more reproducible way than the conventional methods.
Upcoming challenges
- Validation of this one-step liposome production and sterilization process on a larger scale on PGSS pilot equipment located in a clean room enabling aseptic production.
- Optimization of the conditions of production for another formulation encapsulating other active pharmaceutical ingredients.
- Application of the process to the production of Lipid Nanoparticles (LNPs).
Supercritical fluid methods: An alternative to conventional methods to prepare liposomes
Chemical Engineering Journal 383, 123106
| ScienceDirect |
A supercritical fluid technology for liposome production and comparison with the film hydration method
Int. J. Pharm. 592, 120093
| Pubmed |
An innovative one step green supercritical CO(2) process for the production of liposomes co-encapsulating both a hydrophobic and a hydrophilic compound for pulmonary administration
Int. J. Pharm. 627, 122212
| PubMed |
Effects of supercritical carbon dioxide under conditions potentially conducive to sterilization on physicochemical characteristics of a liposome formulation containing apigenin
The Journal of Supercritical Fluids 179, 105418
| ScienceDirect |
Use of supercritical CO(2) for the sterilization of liposomes: Study of the influence of sterilization conditions on the chemical and physical stability of phospholipids and liposomes
Eur. J. Pharm. Biopharm. 183, 112–118
| PubMed |
Development and optimization of a one step process for the production and sterilization of liposomes using supercritical CO(2)
Int. J. Pharm. 651, 123769
| PubMed |

