Analysis of Phospholipids in Bio-Oils and Fats by Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry
Jyrki Viidanoja1)
A sensitive and selective liquid chromatography–electrospray ionization–tandem mass spectrometric (LC–ESIMS/MS) method was developed for simultaneous (semi)-quantification of a very large array of Phospholipid (PL) classes in bio-oils and fats. The method applies Hydrophilic Interaction Liquid Chromatography–scheduled Multiple Reaction Monitoring (HILIC–sMRM) and zwitterionic HPLC column for efficient separation of 14 PL classes (Figure 2) and to obtain a dynamic range of more than six orders of magnitude. Eight PL class-specific internal standards (homologs) and more than 400 scheduled MRMs are employed for the measurement of PLs in a run time of 34 min. The method’s performance was evaluated for vegetable oil, animal fat and algae oil. The averaged within-run precision and between-run precision were ≤10 % for all of the PL classes that had a direct homologue as an internal standard. The method accuracy was generally within 80–120 % for the tested PL analytes in all three sample matrices. The work and the method are described in detail in J. Viidanoja, J. Chromatogr. B 1001 (2015) 140–149.
A variety of oils and fats are typical raw materials for the production of renewable diesel fuels. The raw materials include different types of vegetable oils (1st generation biofuel raw materials), wastes, residues, and side streams (2nd generation biofuel raw materials), such as Animal Fats (AF), Used Cooking Oil (UCO) and Palm Fatty Acid Distillate (PFAD), as well as 3rd generation biofuel raw materials, such as algae oil. In the renewable diesel manufacturing process (Neste NEXBTL diesel), oils and fats are catalytically hydrogenated. The final product is mostly composed of alkanes that originate from fatty acyls of different glycerolipids, mainly mono-,di- and triacylglycerides and free fatty acids. In addition to these lipids, oils and fats contain varying amounts of other lipids, such as phospholipids (PL), which contain phosphorus in their phosphate group. Catalysts are required in the hydrodeoxygenation (HDO) and isomerization steps. Phosphorus is a catalyst poison. Therefore, PLs must be removed from the feedstock using different pretreatment processes, such as degumming and bleaching. The suitability of the feedstock for the manufacturing process and the efficiency of the pretreatment processes can be evaluated by quantifying PLs in each PL class.
Concentrations of PLs in bio-oils and fats can vary by four orders of magnitude, from <1 mg/kg (purified/pretreated materials) to several thousands of milligrams per kilogram (algae oils). In addition, the PL distribution can vary great within and between the phospholipid classes. As a result, the concentration of individual PL species vary by more than six orders of magnitude. To attain such a wide dynamic range in an analytical method, both adjustable sample preparation and detector that provides both wide dynamic range and high selectivity are required.
Bio-oil samples cannot injected directly into the HPLC column, the sample matrix has to be removed first because they contain interfering high amounts of other lipids, mainly acylglycerides and free fatty acids. Generally, it is done with Solid Phase Extraction (SPE) and by retaining the PLs on the SPE column while the matrix is washed away. Diprotic PLs, phosphatidic and lysophosphatidic acid may exist in oils in Mg and Ca metal chelate forms. To ensure that they are retained in SPE and later in chromatography as expected, citric acid is added to the sample to break the chelates prior to SPE. Various sample dilution steps are performed before SPE to avoid overloading the SPE cartridge and detector with PLs and other highly retaining lipids. The correct dilution factor for sample dilution can be determined based on the initial phosphorus content of the sample that is determined separately with another method. The Internal standard (IS) concentration in the sample solution is kept constant, irrespective the dilution factor because method performance (sensitivity) may otherwise deteriorate.
This also allows IS signal intensity to be used as Quality Control (QC) metrics for SPE recovery and LC-MS performance. This is especially important when the method is used for the analysis of diverse range of sample matrices of variable complexity and lipid composition, several of them not necessarily included in the method validation. In addition, IS intensity is preferred to be similar in the sample and in calibration standards even if calibration curves are seemingly linear.
PLs in oils and fats have traditionally been measured using high-performance liquid chromatography (HPLC) with ultraviolet (UV) or evaporative light scattering (ELSD) detection. These techniques are excellent for high concentrations and for the specific analytical problems for which they were created. However, they have limited selectivity and sensitivity and cannot provide universal solution for wide range of lipid profiles and concentrations.
Liquid chromatography tandem mass spectrometry (LC–MS/MS) and direct-infusion tandem mass spectrometry (MS/MS), the so-called shotgun lipidomics approach, offer more universal solutions for PL analytics. Due to the MS/MS overlap, certain PL species of phosphatidylcholines (PC) and sphingomyelins (SM) may not be distinguished from each other without LC. LC–MS/MS also yields better sensitivity and detection of minor components than the shotgun approach due to the sequential ionization of the analytes. Measurement sensitivity is further improved by removing most of the sample matrix with SPE prior to LC–MS/MS (matrix effects reduced or omitted). In order to monitor hundreds of MS/MS transitions in a single LC-MS method and simultaneously to have reasonable MS/MS sensitivity (MS/MS duty cycle), MS/MS operation has to be scheduled. In scheduled Multiple Reaction Monitoring (sMRM) individual MS/MS transitions are monitored only within time window where compounds elute out of the LC column.
Because PLs are polar and non-volatile, electrospray ionization (ESI) has to be employed in conjunction with MS. In practice, ESI requires internal standards (ISs) for quantitative analysis due to its susceptibility to matrix effects and temporal signal variation. Bio-oils can typically contain tens to hundreds of PL species, and their identity varies between sample types. It is practically impossible to have separate calibration curves and internal standards for each PL species. However, individual PL species possess similar response factors within acyl carbon and double bond range bio-oils typically have. Therefore, calibration with PL standards which acyl carbon number is similar to the analytes, leads to quantitative results, when the experimental conditions are carefully selected.
By default, calibration standards should be run and calibration curves generated in LC-MS every day samples are being run. However, this is not needed if Relative Response Factors (RRFs) (i.e. the ratio of response of the analyte to the IS) do not change over time. This is more likely the more similar the analyte and IS are. At best the IS is a stable isotope analog of the analyte but applying closely related homolog as an IS can also lead to relatively stable RRFs. In that case, instead of reproducing calibration curves every day, a sound calibration may be applied. It is created by running calibration standards on multiple days and combining them into a single calibration. Stability of the RRFs and validity of the approach should be evaluated for example by studying how much daily calibration curves deviate from each other (accuracy of calibration). If approach is considered accurate enough validity of fixed calibration is verified for each run by running reference sample and confirming that measured concentration is within acceptance limits from the value assigned when calibration was generated or method was validated. The need for applying fixed calibration in case of PLs stems from the challenges in preparing, ensuring and maintaining accurate and stable calibration solutions. These practical issues can be a larger risk for maintaining accurate calibration than inherent stability of RRFs.
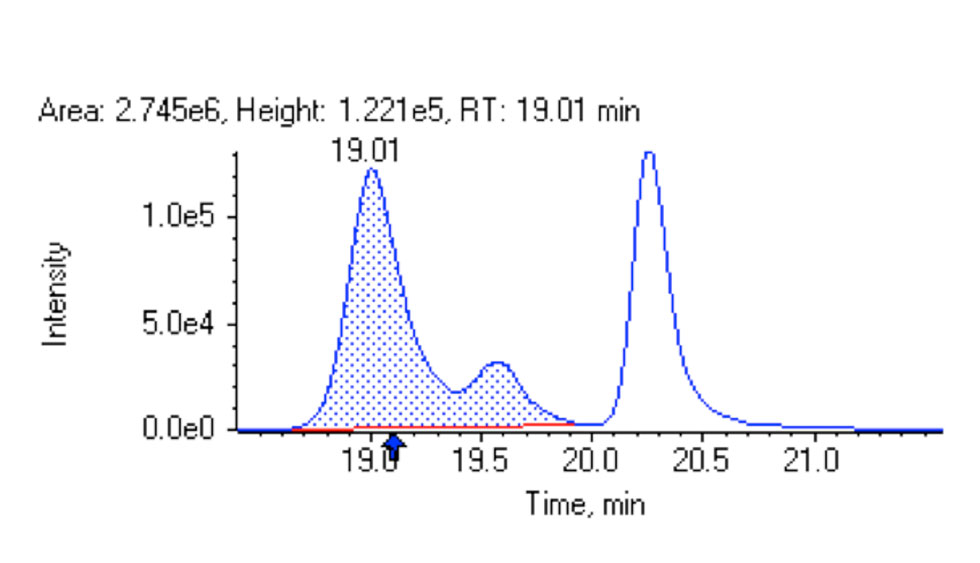
Figure 1: Good peak shape and chromatographic separation was obtained for three isomers of LPA 20:4 (457/153 MS/MS selected ion chromatogram).
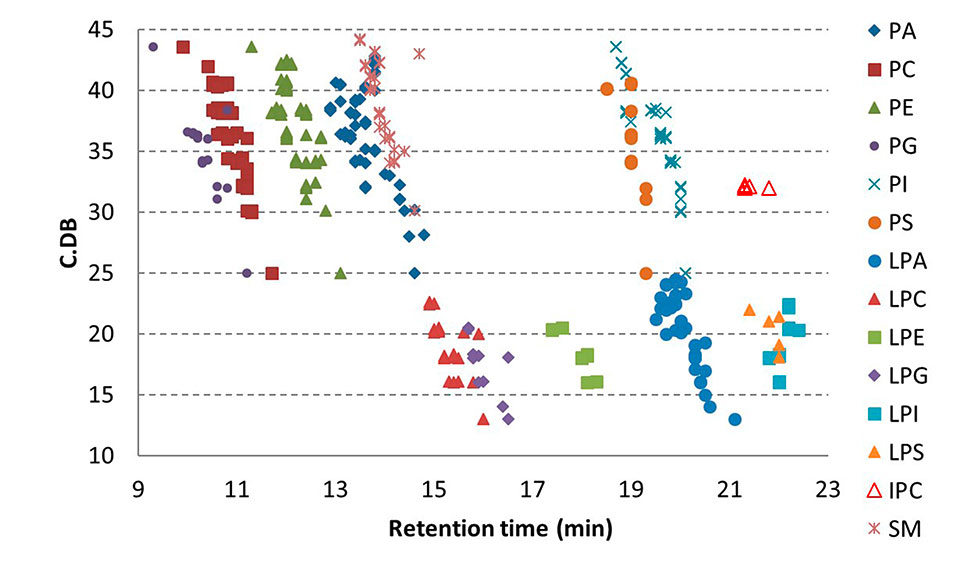
Figure 2: Retention times of phospholipids as a function of the amount of fatty acyl carbons (C) and double bonds (DB) in the molecule.
In practice, it is very challenging to obtain a good chromatographic peak shape and chromatographic separation simultaneously for a large array of PL classes. One of the most challenging PL classes for obtaining a symmetric peak shape without tailing is LPA (Figure 1).
Excellent separation of 14 PL classes (Phosphatidic acid (PA), Phosphatidylcholine (PC), Phosphatidylethanolamine (PE), Phosphatidylglycerol (PG), Phosphatidylinositol (PI), Phosphatidylserin (PS), Lyso-PA, (LPA), Lyso-PC (LPC), Lyso-PE (LPE), Lyso-PG (LPG), Lyso-PI (LPI), Lyso-PS (LPS), Inositolphosphoceramide (IPC) and Sphingomyelin (SM)) was obtained with the HILIC method (Figure 2).
In addition to good peak shape, it is important that the PL classes that produce the same fragment ion (Q3 ion) or the same neutral loss (60 amu for PC and SM) do not coelute because of an increased risk of measurement interference. This requirement was fulfilled by the method for all of the PL classes, except for PIs that co-elute with LPA (Figure 1). However, because LPA is a lyso species and has a small phospholipid head group, while PI has a large head group, these two PL classes have very different Q1 masses and can be discerned from each other based on Q1 mass.
In the Hydrophilic Interaction Liquid Chromatography (HILIC), retention of PLs on the HPLC column is based on interactions between the polar PL head groups and polar stationary phase, while the structure of fatty acyls, including the amount of fatty acyl carbons and double bonds, plays a minor role in the retention. Thus, the PLs of the same PL class elute out of the column at approximately the same time interval. The great benefit of this is that it enables the use of PL class-specific internal standards that have the same head group and number of fatty acyl groups as the analytes and which elute within the same time range as the PL analytes of the class. Therefore, it can be expected that the analytes and the lipid class-specific IS experience similar ionization conditions (ESI matrix effects and temporal signal variation). The resulting signal normalization by IS is the basis of quantitative measurement, when ESI is applied.
Many PL classes can be measured either in the positive or negative mode by monitoring the protonated or deprotonated molecules or various adducts. The negative ion mode was selected for the method because it allows for the measurement of all of the PL classes, including PA and LPA, in the same run without applying polarity switching. The measurement of PC, PE, LPC and SM would have been more sensitive in the positive mode, but because PC and LPC were the dominant PL classes in almost all of the tested bio-oils, measurement of PC or LPC in the positive mode would have resulted in larger dilution factors to avoid detector saturation, which would have also compromised the sensitivity of the measurement for several other PL classes.
Before selecting the IS and IS MS/MS transitions to be used in the method a large number of different bio-oils and fats were analyzed to identify commercially available IS candidates that have negligible ion background in sample MS/MS spectra (within the target sMRM window). This is critical to avoid (negative) bias in quantification, due to sample component signal being added to the IS signal. This excluded the PLs with 17:0/17:0 and lyso PLs 17:0 fatty acyl chains that are typically used as IS in lipidomic experiments because species with the same Q1 and Q3 masses were detected in several sample matrices. One or two IS candidates were selected per PL class for method accuracy evaluation. Due to the limited availability of IS candidate materials and to limit the complexity and the number of IS in the method, the IS of PE, PG and PI were evaluated as the IS for the corresponding lyso species. PC IS was evaluated as the IS for SM. It was acknowledged that as a result of this, the measurement of these lyso species might not be as quantitative as the measurement of the other PL classes.
Further details on the method parameters, method development and validation can be found in J. Viidanoja, J. Chromatogr. B 1001 (2015) 140–149. Briefly, the analytical performance and suitability of the method for the intended purpose were assessed by method validation which included studying the linearity, precision (within run and between runs), accuracy and LLOQ separately for three different sample matrices, namely: Soya Bean Oil (SBO), Animal Fat (AF) and Nannochloropsis oculata algae oil (1st, 2nd and 3rd generation biofuel raw material, respectively). Precision was determined by analyzing four replicates of each sample on three days using two different injection volumes. Accuracy was determined by spiking studies (four concentration levels) using from one to two different ISs and the same analytes as those used for instrument calibration. The validation was termed partial because full validation was impossible due to practical limitations (only limited number of pure analytes standards are available and can be included in the validation. The validation could be done only with limited set of sample matrices).
The calibration curves were found to be linear over three orders of magnitude. The averaged within-run precision and between-run precision were generally ≤10 % for all of the PL classes that had a direct homologue as an IS and the concentration was ≥1 mg P/kg. Accuracy was generally within 80–120 % for all the three sample matrices, indicating that ISs and analytes behaved similarly in SPE and ISs corrected possible ESI matrix effects. Based on these results it can be concluded that the hydrophilic interaction liquid chromatography–tandem mass spectrometry method is suitable for quantitative analysis of broad range of phospholipids from diverse and complex sample matrices.

